| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Abacavir sulfate (ABC) targets HIV-1 reverse transcriptase (EC50 = 0.08 μM in HIV-1-infected human PBMCs; Ki = 0.01 μM for recombinant HIV-1 reverse transcriptase) [4]
Abacavir sulfate (ABC) inhibits prostate cancer cell proliferation via targeting cellular DNA synthesis (IC50 = 15 μM for LNCaP cells; IC50 = 18 μM for PC-3 cells) [1] Abacavir sulfate (ABC) suppresses medulloblastoma cell viability (IC50 = 20 μM for DAOY cells; IC50 = 22 μM for D283 cells) [3] |
|---|---|
| 体外研究 (In Vitro) |
在前列腺癌细胞系中,阿巴卡韦(15 和 150 μM,0-120 小时)硫酸盐可减少细胞增殖、修饰 LINE-1 mRNA 表达、改变细胞周期进程并促进衰老[1]。阿巴卡韦硫酸盐(15 和 150 μM,18 小时)可大大减少细胞迁移并抑制细胞侵袭[1]。硫酸阿巴卡韦诱导脂肪细胞凋亡[4]。
硫酸阿巴卡韦(Abacavir sulfate, ABC) 抑制人前列腺癌细胞系LNCaP和PC-3的增殖,15 μM和18 μM浓度下分别使细胞活力降低50%;20 μM浓度下膜联蛋白V阳性细胞比例增加30%,诱导细胞凋亡 [1] 硫酸阿巴卡韦(Abacavir sulfate, ABC) 下调LNCaP细胞中抗凋亡蛋白Bcl-2的表达,上调促凋亡蛋白Bax的表达(western blot分析)[1] 硫酸阿巴卡韦(Abacavir sulfate, ABC) 与放疗和地西他滨联合使用时,对髓母细胞瘤细胞DAOY和D283表现出协同细胞毒性,10 μM浓度联合治疗使细胞存活率降低70%,而单药治疗仅降低30% [3] 硫酸阿巴卡韦(Abacavir sulfate, ABC) 使人脂肪细胞中的线粒体DNA(mtDNA)水平较司他夫定处理组升高1.8倍,并减少45%的脂肪细胞凋亡 [4] 硫酸阿巴卡韦(Abacavir sulfate, ABC) 在浓度高达50 μM时,对正常人前列腺上皮细胞(PrEC)的活力无显著影响 [1] |
| 体内研究 (In Vivo) |
阿巴卡韦硫酸盐(0-7.5 μg/mL,100 μL,阴囊内注射;100 和 200 mg/kg,口服;4 小时)硫酸盐可剂量依赖性地增加血栓形成[2]。在携带髓母细胞瘤的高危小鼠中,硫酸阿巴卡韦(50 mg/kg/d;腹腔注射;14 天)联合 0.1 mg/kg/d 地西他滨可提高生存率[3]。
硫酸阿巴卡韦(Abacavir sulfate, ABC) 以100 mg/kg/天的剂量口服给药C57BL/6小鼠14天后,动脉血栓形成发生率增加60% [2] 硫酸阿巴卡韦(Abacavir sulfate, ABC) 与放疗(2 Gy)和地西他滨(0.2 mg/kg)联合使用时,使髓母细胞瘤荷瘤裸鼠的存活率提高40%,肿瘤体积较对照组减少55% [3] 硫酸阿巴卡韦(Abacavir sulfate, ABC) 改善先前经司他夫定治疗小鼠的脂肪萎缩,口服50 mg/kg/天8周后,附睾脂肪垫重量增加35% [4] 硫酸阿巴卡韦(Abacavir sulfate, ABC) 使小鼠脂肪细胞凋亡减少38%,脂肪组织mtDNA水平较司他夫定治疗组升高2.1倍 [4] |
| 酶活实验 |
HIV-1逆转录酶抑制实验:制备包含重组HIV-1逆转录酶、多聚(rA)-寡聚(dT)模板引物和[3H]-dGTP的反应体系。加入系列稀释浓度的硫酸阿巴卡韦(Abacavir sulfate, ABC),在37°C下孵育90分钟。用三氯乙酸终止反应,通过玻璃纤维滤膜过滤,测定放射性强度以计算酶抑制效率 [4]
细胞DNA合成实验:在24孔板中培养LNCaP细胞,用硫酸阿巴卡韦(Abacavir sulfate, ABC)(5–50 μM)处理24小时后,加入[3H]-胸腺嘧啶核苷孵育4小时。收获细胞,用冷PBS洗涤,测定放射性强度以评估DNA合成抑制情况 [1] |
| 细胞实验 |
细胞增殖测定[1]
细胞类型: PC3、LNCaP 和 WI-38 测试浓度: 15 和 150 μM 孵育持续时间:0、24、48、72 和 96 小时 实验结果:证明对 PC3 和 LNCaP 具有剂量依赖性生长抑制作用。 细胞周期分析[1] 细胞类型: PC3 和 LNCaP 测试浓度: 150 μM 孵育持续时间:0、18、24、48、72、96和120小时 实验结果:导致PC3和LNCaP细胞中S期细胞的大量积累,并且在 PC3 细胞中观察到 G2/M 期增量。 细胞迁移测定 [1] 细胞类型: PC3 和 LNCaP 测试浓度: 15 和 150 μM 孵育时间: 18 小时 实验结果:细胞迁移显着减少。细胞侵袭测定[1] 细胞类型: PC3 和 LNCaP 测试浓度: 15 和 150 μM 孵育时间:18小时 实验结果:显着抑制细胞观察。 前列腺癌细胞增殖与凋亡实验:在96孔板中以3×104个细胞/孔接种LNCaP和PC-3细胞。用硫酸阿巴卡韦(Abacavir sulfate, ABC)(1–50 μM)处理72小时。通过MTT法评估细胞活力以计算IC50;膜联蛋白V-FITC/PI染色后流式细胞术分析凋亡率;提取蛋白通过western blot检测Bcl-2和Bax的表达 [1] 髓母细胞瘤细胞联合治疗实验:在96孔板中以2×104个细胞/孔接种DAOY和D283细胞。用硫酸阿巴卡韦(Abacavir sulfate, ABC)(5–25 μM)预处理2小时后,进行放疗(2 Gy)和地西他滨(0.5 μM)处理。孵育5天后,采用克隆形成实验测定细胞存活率,计算联合指数(CI)[3] 脂肪细胞mtDNA与凋亡实验:从皮下脂肪组织中分离人脂肪细胞,在6孔板中以1×106个细胞/孔接种。用硫酸阿巴卡韦(Abacavir sulfate, ABC)(10 μM)或司他夫定(10 μM)处理14天。提取总DNA,通过实时PCR定量mtDNA水平;膜联蛋白V-FITC染色检测凋亡率 [4] |
| 动物实验 |
Animal/Disease Models: Male mice (9-weeks old, 22-30 g) - wild-type (WT) C57BL/ 6 or homozygous knockout (P2rx7 KO, B6.129P2-P2rx7tm1Gab/J)[2]
Doses: 2.5, 5 and 7.5 μg/mL, 100 μL or 100 and 200 mg/kg Route of Administration: Intrascrotal or oral administration for 4 h Experimental Results: Dose-dependently promoted thrombus formation. Animal/Disease Models: NSGTM mice, patient-derived xenograft (PDX) cells of non-WNT/non-SHH, Group 3 and of SHH/ TP53-mutated medulloblastoma[3] Doses: 50 mg/kg/ d with 0.1 mg/kg/d Decitabine Route of Administration: intraperitoneal (ip)injection, daily for 14 days Experimental Results: Inhibited tumor growth and enhanced mouse survival. Arterial thrombosis mouse assay: Male C57BL/6 mice (8–10 weeks old) are administered Abacavir sulfate (ABC) via oral gavage at 50 or 100 mg/kg/day for 14 days. The drug is formulated in 0.5% methylcellulose. On day 15, mice undergo ferric chloride-induced carotid artery injury, and blood flow is monitored for 30 min to determine thrombosis incidence [2] Medulloblastoma mouse model assay: Nude mice (6–8 weeks old) are intracranially implanted with DAOY medulloblastoma cells (1×105 cells/mouse). Seven days post-implantation, mice receive combined treatment: Abacavir sulfate (ABC) (30 mg/kg/day, oral gavage), radiotherapy (2 Gy, once weekly for 3 weeks), and decitabine (0.2 mg/kg/day, intraperitoneal injection). Tumor volume is measured every 3 days via MRI, and survival rate is recorded for 60 days [3] Lipoatrophy mouse model assay: Mice are first treated with stavudine (50 mg/kg/day, oral) for 12 weeks to induce lipoatrophy. Then, stavudine is replaced with Abacavir sulfate (ABC) (50 mg/kg/day, oral) for another 8 weeks. At study end, epididymal and subcutaneous fat pads are harvested for weight measurement; adipose tissue is analyzed for mtDNA levels (real-time PCR) and apoptotic cells (TUNEL staining) [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration of a 600-mg dose of radiolabeled abacavir, 82.2% of the dose is excreted in urine and 16% of the dose is excreted in feces. The 5-carboxylic acid metabolite, 5-glucuronide metabolite, and unchanged abacavir accounted for 30, 36, and 1.2%, respectively, of recovered radioactivity in urine; unidentified minor metabolites accounted for 15% of recovered radioactivity in urine. It is not known whether abacavir is distributed into human milk; the drug is distributed into milk in rats. Abacavir crosses the placenta in rats. The oral bioavailability of abacavir is high with or without food; the CSF-to-plasma AUC ratio is approximately 0.3. For more Absorption, Distribution and Excretion (Complete) data for ABACAVIR SULFATE (7 total), please visit the HSDB record page. Metabolism / Metabolites Abacavir is partially metabolized by alcohol dehydrogenase (to form the 5'-carboxylic acid) and glucuronidation (to form the 5'-glucuronide). The metabolic fate of abacavir has not been fully determined, but the drug is metabolized in the liver. Abacavir is metabolized by alcohol dehydrogenase to form the 5-carboxylic acid and by glucuronyltransferase to form the 5-glucuronide; these metabolites do not appear to have any antiviral activity. Any involvement of cytochrome p450 isoenzymes in the metabolism of abacavir is limited. Intracellularly, abacavir is phosphorylated to abacavir monophosphate by adenosine phosphotransferase; abacavir monophosphate is then converted to carbovir monophosphate in a reaction catalyzed by cytosolic enzymes and then to carbovir triphosphate by cellular kinases. Intracellular (host cell) conversion of abacavir to carbovir triphosphate is necessary for the antiviral activity of the drug. The in vitro intracellular half-life of carbovir triphosphate in CD4+ CEM cells is 3.3 hours. Biological Half-Life The in vitro intracellular half-life of carbovir triphosphate /SRP: a metabolite of abacavir sulfate,/ in CD4+ CEM cells is 3.3 hours. The plasma elimination half-life of abacavir following a single oral dose (given as abacavir sulfate) is about 1.5 hours. In HIV-infected children 3 months to 13 years of age who received 8 mg/kg of abacavir every 12 hours (given as an oral solution containing abacavir sulfate), steady-state plasma elimination half-life averaged 1.3 hours and was essentially the same as that reported after a single dose. Following oral administration of a single 300-mg dose of abacavir to an individual with renal failure (glomerular filtration rate less than 10 mL/minute) undergoing peritoneal dialysis, the plasma elimination half-life of the drug was 1.33 hours. Abacavir sulfate (ABC) has an oral bioavailability of 76% in humans [4] Abacavir sulfate (ABC) is rapidly absorbed in humans, reaching peak plasma concentrations (Cmax) of 3.0 μg/mL at a Tmax of 0.8 h after oral administration of 300 mg [4] The area under the plasma concentration-time curve (AUC0–24h) of Abacavir sulfate (ABC) in humans is 8.6 μg·h/mL at 300 mg twice daily [4] Abacavir sulfate (ABC) has a volume of distribution (Vd) of 0.8 L/kg in humans [4] The plasma elimination half-life (t1/2) of Abacavir sulfate (ABC) in humans is 1.5 h [4] Abacavir sulfate (ABC) is metabolized primarily by alcohol dehydrogenase and glucuronyl transferase in the liver [4] Renal excretion accounts for 1.2% of the administered dose of Abacavir sulfate (ABC) in humans [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Abacavir appears in breastmilk in small quantities. Very little information is available on the safety of its use during breastfeeding. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended. ◉ Effects in Breastfed Infants An HIV-positive mother took a combination tablet containing dolutegravir 50 mg, abacavir sulfate 600 mg and lamivudine 300 mg (Triumeq) once daily. Her infant was exclusively breastfed for about 30 weeks and partially breastfed for about 20 weeks more. No obvious side effects were noted. ◉ Effects on Lactation and Breastmilk Gynecomastia has been reported among men receiving highly active antiretroviral therapy. Gynecomastia is unilateral initially, but progresses to bilateral in about half of cases. No alterations in serum prolactin were noted and spontaneous resolution usually occurred within one year, even with continuation of the regimen. Some case reports and in vitro studies have suggested that protease inhibitors might cause hyperprolactinemia and galactorrhea in some male patients, although this has been disputed. The relevance of these findings to nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Interactions Concurrent use /of ethanol/ with abacavir may result in increased concentrations and half-life of abacavir as a result of competition for common metabolic pathways via alcohol dehydrogenase. Methadone clearance increased 22% in patients stabilized on oral methadone maintenance therapy who started abacavir therapy with 600 mg twice daily; increase in clearance will not be clinically significant in the majority of patients; methadone dosage increase may be required in a small number of patients. Abacavir sulfate (ABC) induces arterial thrombosis in mice at doses ≥ 50 mg/kg/day [2] Abacavir sulfate (ABC) has a plasma protein binding rate of < 5% in humans [4] In humans, the most common adverse events include nausea (11%), headache (9%), and fatigue (7%); severe hypersensitivity reaction occurs in ~5% of patients [4] Abacavir sulfate (ABC) does not cause significant mitochondrial toxicity in human adipocytes, unlike stavudine [4] The oral LD50 of Abacavir sulfate (ABC) in mice is > 2000 mg/kg [4] |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Abacavir is indicated, in combination with other agents, for treatment of HIV-1 infection. /Included in US product labeling/ Drug Warnings A unique and potentially fatal hypersensitivity reaction occurs in 2% to 5% of patients receiving abacavir. Symptoms typically occur within the first six weeks of therapy and include fever, rash, nausea, malaise, and respiratory complaints, in various combinations. Symptoms initially may be mild but increase in severity with continued administration. Discontinuation of the medication usually resolves all signs and symptoms, but rechallenge may cause rapid onset of severe reactions, hypotension, and death. Once an abacavir hypersensitivity reaction is suspected or confirmed, it is recommended that the patient never by rechallenged with abacavir. The major toxicity associated with abacavir therapy is potentially life-threatening hypersensitivity reactions. In clinical studies, hypersensitivity reactions have been reported in approximately 5% of adult and pediatric patients receiving abacavir in conjunction with lamivudine and zidovudine. Fatalities related to hypersensitivity reactions to abacavir have been reported. Manifestations of hypersensitivity usually are apparent within the first 6 weeks of abacavir therapy, but may occur at any time during therapy. Severe hypersensitivity reactions are likely to recur within hours following rechallenge in patients with a prior history of hypersensitivity to the drug, and these reactions may include life-threatening hypotension and death. The most severe hypersensitivity reactions reported to date have been in individuals who were rechallenged with abacavir after a previous hypersensitivity reaction to the drug. There also have been reports of severe or fatal hypersensitivity reactions occurring after abacavir was reintroduced in patients with no identified history of abacavir hypersensitivity or with unrecognized manifestations of hypersensitivity to the drug. Although these patients had discontinued abacavir for reasons unrelated to hypersensitivity (e.g., interruption in drug supply, discontinuance of abacavir during treatment for other medical conditions), some may have had symptoms present before discontinuance of the drug that were consistent with hypersensitivity but were attributed to other medical conditions (e.g., acute onset respiratory disease, gastroenteritis, adverse reactions to other drugs). Most of the hypersensitivity reactions reported following reintroduction of abacavir in these patients were indistinguishable from hypersensitivity reactions associated with abacavir rechallenge (i.e., short time to onset, increased severity of symptoms, poor outcome including death).Hypersensitivity reactions can occur within hours after abacavir is reintroduced; however, in some cases, these reactions occurred days to weeks following reintroduction of the drug. Lactic acidosis and severe hepatomegaly with steatosis (sometimes fatal) have been reported rarely in patients receiving abacavir and also have been reported in patients receiving dideoxynucleoside reverse transcriptase inhibitors. Most reported cases have involved women; obesity and long-term therapy with a nucleoside reverse transcriptase inhibitor also may be risk factors. Increased serum concentrations of Gamma-glutamyltransferase (GGT, GGPT) have been reported in patients receiving abacavir. Hypersensitivity reactions reported in patients receiving abacavir are characterized by the appearance of manifestations indicating involvement of multiple organ and body systems; these reactions have occurred in association with anaphylaxis, liver failure, renal failure, hypotension, and death. The most frequent manifestations of hypersensitivity reactions to abacavir include fever, rash, fatigue, GI symptoms such as nausea, vomiting, diarrhea, and abdominal pain, and respiratory symptoms such as pharyngitis, dyspnea, and cough. Other signs and symptoms include malaise, lethargy, myalgia, myolysis, headache, arthralgia, edema, paresthesia, lymphadenopathy, and mucous membrane lesions (e.g., conjunctivitis, mouth ulceration). Respiratory symptoms, including cough, dyspnea, and pharyngitis, have been reported in approximately 20% of patients with hypersensitivity reactions to abacavir. Fatalities have occurred in patients who developed hypersensitivity reactions in which the initial presentation included respiratory symptoms; some patients who experienced fatal hypersensitivity reactions were initially diagnosed as having an acute respiratory disease (pneumonia, bronchitis, flu-like illness). Hypersensitivity reactions can occur without rash; if rash occurs, it usually is maculopapular or urticarial, but may be variable in appearance. Laboratory abnormalities reported in patients experiencing a hypersensitivity reaction to abacavir include lymphopenia and increases in serum concentrations of liver enzymes, creatine kinase (CK, creatine phosphokinase, CPK), or creatinine. For more Drug Warnings (Complete) data for ABACAVIR SULFATE (17 total), please visit the HSDB record page. infection in adults and children [4] Abacavir sulfate (ABC) exerts its antiviral effect by intracellular conversion to abacavir triphosphate, which competes with deoxyguanosine triphosphate (dGTP) for incorporation into viral DNA, terminating HIV-1 DNA synthesis [4] Abacavir sulfate (ABC) exhibits anticancer activity against prostate cancer and medulloblastoma, potentially via inhibiting cellular DNA replication [1][3] Abacavir sulfate (ABC) is recommended as an alternative to stavudine in HIV treatment regimens to reduce lipoatrophy and mitochondrial toxicity [4] Abacavir sulfate (ABC) was approved by the FDA in 1998 for HIV-1 treatment [4] |
| 分子式 |
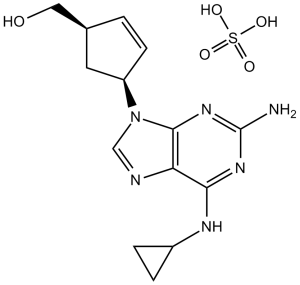
C14H18N6O.1/2H2O4S
|
|
|---|---|---|
| 分子量 |
335.35
|
|
| 精确质量 |
670.275
|
|
| CAS号 |
188062-50-2
|
|
| 相关CAS号 |
Abacavir;136470-78-5;Abacavir monosulfate;216699-07-9;Abacavir hydrochloride;136777-48-5
|
|
| PubChem CID |
441384
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.9±0.1 g/cm3
|
|
| 沸点 |
636ºC at 760 mmHg
|
|
| 熔点 |
222-225ºC
|
|
| 闪点 |
338.4ºC
|
|
| 折射率 |
1.851
|
|
| LogP |
0.74
|
|
| tPSA |
153.63
|
|
| 氢键供体(HBD)数目 |
8
|
|
| 氢键受体(HBA)数目 |
16
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
47
|
|
| 分子复杂度/Complexity |
496
|
|
| 定义原子立体中心数目 |
4
|
|
| SMILES |
C1CC1NC2=C3C(=NC(=N2)N)N(C=N3)[C@@H]4C[C@@H](C=C4)CO.C1CC1NC2=C3C(=NC(=N2)N)N(C=N3)[C@@H]4C[C@@H](C=C4)CO.OS(=O)(=O)O
|
|
| InChi Key |
MBFKCGGQTYQTLR-SCYNACPDSA-N
|
|
| InChi Code |
InChI=1S/C14H18N6O.H2O4S/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21;1-5(2,3)4/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19);(H2,1,2,3,4)/t8-,10+;/m1./s1
|
|
| 化学名 |
[(1S,4R)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol sulfuric acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.45 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.45 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.45 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10 mg/mL (29.82 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9820 mL | 14.9098 mL | 29.8196 mL | |
| 5 mM | 0.5964 mL | 2.9820 mL | 5.9639 mL | |
| 10 mM | 0.2982 mL | 1.4910 mL | 2.9820 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Mycophenolate Mofetil and Abacavir Treatment in HIV Patients With Failed Anti-HIV Treatment
CTID: NCT00021489
Phase: Phase 2 Status: Withdrawn
Date: 2021-11-01