| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |

| 体外研究 (In Vitro) |
在前列腺癌细胞系中,阿巴卡韦盐酸盐(15 和 150 μM,0-120 小时)抑制细胞生长,修饰 LINE-1 mRNA 的表达,影响细胞周期的发育,并促进衰老[1]。盐酸阿巴卡韦(15 和 150 μM,18 小时)极大地抑制细胞迁移和侵袭[1]。盐酸阿巴卡韦可诱导脂肪细胞凋亡[4]。
|
|---|---|
| 体内研究 (In Vivo) |
盐酸阿巴卡韦在 100 和 200 mg/kg(口服)时剂量依赖性地促进血栓形成; 4 小时[2]。 0.1 mg/kg/d 地西他滨和 50 mg/kg/d 盐酸阿巴卡韦的组合(腹腔注射;14 天)可改善携带髓母细胞瘤的高危小鼠的存活率[3]。
|
| 动物实验 |
Animal/Disease Models: Male mice (9-weeks old, 22-30 g) - wild-type (WT) C57BL/6 or homozygous knockout (P2rx7 KO, B6.129P2-P2rx7tm1Gab /J)[2]
Doses: Route 1: 2.5, 5, and 7.5 μg/mL, 100 μL Route 2: 100 and 200 mg/kg Route of Administration: Intrascrotal or oral administration for 4 h Experimental Results: Dose-dependently promoted thrombus formation. Animal/Disease Models: NSGTM mice, patient-derived xenograft (PDX) cells of non-WNT/non-SHH, Group 3 and of SHH/ TP53-mutated medulloblastoma[3] Doses: 50 mg/kg/d with 0.1 mg/kg/ d Decitabine Route of Administration: intraperitoneal (ip)injection, daily for 14 days Experimental Results: Inhibited tumor growth and enhanced mouse survival. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration of a 600-mg dose of radiolabeled abacavir, 82.2% of the dose is excreted in urine and 16% of the dose is excreted in feces. The 5-carboxylic acid metabolite, 5-glucuronide metabolite, and unchanged abacavir accounted for 30, 36, and 1.2%, respectively, of recovered radioactivity in urine; unidentified minor metabolites accounted for 15% of recovered radioactivity in urine. It is not known whether abacavir is distributed into human milk; the drug is distributed into milk in rats. Abacavir crosses the placenta in rats. The oral bioavailability of abacavir is high with or without food; the CSF-to-plasma AUC ratio is approximately 0.3. For more Absorption, Distribution and Excretion (Complete) data for ABACAVIR SULFATE (7 total), please visit the HSDB record page. Metabolism / Metabolites Abacavir is partially metabolized by alcohol dehydrogenase (to form the 5'-carboxylic acid) and glucuronidation (to form the 5'-glucuronide). The metabolic fate of abacavir has not been fully determined, but the drug is metabolized in the liver. Abacavir is metabolized by alcohol dehydrogenase to form the 5-carboxylic acid and by glucuronyltransferase to form the 5-glucuronide; these metabolites do not appear to have any antiviral activity. Any involvement of cytochrome p450 isoenzymes in the metabolism of abacavir is limited. Intracellularly, abacavir is phosphorylated to abacavir monophosphate by adenosine phosphotransferase; abacavir monophosphate is then converted to carbovir monophosphate in a reaction catalyzed by cytosolic enzymes and then to carbovir triphosphate by cellular kinases. Intracellular (host cell) conversion of abacavir to carbovir triphosphate is necessary for the antiviral activity of the drug. The in vitro intracellular half-life of carbovir triphosphate in CD4+ CEM cells is 3.3 hours. Biological Half-Life The in vitro intracellular half-life of carbovir triphosphate /SRP: a metabolite of abacavir sulfate,/ in CD4+ CEM cells is 3.3 hours. The plasma elimination half-life of abacavir following a single oral dose (given as abacavir sulfate) is about 1.5 hours. In HIV-infected children 3 months to 13 years of age who received 8 mg/kg of abacavir every 12 hours (given as an oral solution containing abacavir sulfate), steady-state plasma elimination half-life averaged 1.3 hours and was essentially the same as that reported after a single dose. Following oral administration of a single 300-mg dose of abacavir to an individual with renal failure (glomerular filtration rate less than 10 mL/minute) undergoing peritoneal dialysis, the plasma elimination half-life of the drug was 1.33 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Abacavir appears in breastmilk in small quantities. Very little information is available on the safety of its use during breastfeeding. Achieving and maintaining viral suppression with antiretroviral therapy decreases breastfeeding transmission risk to less than 1%, but not zero. Individuals with HIV who are on antiretroviral therapy with a sustained undetectable viral load and who choose to breastfeed should be supported in this decision. If a viral load is not suppressed, banked pasteurized donor milk or formula is recommended. ◉ Effects in Breastfed Infants An HIV-positive mother took a combination tablet containing dolutegravir 50 mg, abacavir sulfate 600 mg and lamivudine 300 mg (Triumeq) once daily. Her infant was exclusively breastfed for about 30 weeks and partially breastfed for about 20 weeks more. No obvious side effects were noted. ◉ Effects on Lactation and Breastmilk Gynecomastia has been reported among men receiving highly active antiretroviral therapy. Gynecomastia is unilateral initially, but progresses to bilateral in about half of cases. No alterations in serum prolactin were noted and spontaneous resolution usually occurred within one year, even with continuation of the regimen. Some case reports and in vitro studies have suggested that protease inhibitors might cause hyperprolactinemia and galactorrhea in some male patients, although this has been disputed. The relevance of these findings to nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Interactions Concurrent use /of ethanol/ with abacavir may result in increased concentrations and half-life of abacavir as a result of competition for common metabolic pathways via alcohol dehydrogenase. Methadone clearance increased 22% in patients stabilized on oral methadone maintenance therapy who started abacavir therapy with 600 mg twice daily; increase in clearance will not be clinically significant in the majority of patients; methadone dosage increase may be required in a small number of patients. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Abacavir is indicated, in combination with other agents, for treatment of HIV-1 infection. /Included in US product labeling/ Drug Warnings A unique and potentially fatal hypersensitivity reaction occurs in 2% to 5% of patients receiving abacavir. Symptoms typically occur within the first six weeks of therapy and include fever, rash, nausea, malaise, and respiratory complaints, in various combinations. Symptoms initially may be mild but increase in severity with continued administration. Discontinuation of the medication usually resolves all signs and symptoms, but rechallenge may cause rapid onset of severe reactions, hypotension, and death. Once an abacavir hypersensitivity reaction is suspected or confirmed, it is recommended that the patient never by rechallenged with abacavir. The major toxicity associated with abacavir therapy is potentially life-threatening hypersensitivity reactions. In clinical studies, hypersensitivity reactions have been reported in approximately 5% of adult and pediatric patients receiving abacavir in conjunction with lamivudine and zidovudine. Fatalities related to hypersensitivity reactions to abacavir have been reported. Manifestations of hypersensitivity usually are apparent within the first 6 weeks of abacavir therapy, but may occur at any time during therapy. Severe hypersensitivity reactions are likely to recur within hours following rechallenge in patients with a prior history of hypersensitivity to the drug, and these reactions may include life-threatening hypotension and death. The most severe hypersensitivity reactions reported to date have been in individuals who were rechallenged with abacavir after a previous hypersensitivity reaction to the drug. There also have been reports of severe or fatal hypersensitivity reactions occurring after abacavir was reintroduced in patients with no identified history of abacavir hypersensitivity or with unrecognized manifestations of hypersensitivity to the drug. Although these patients had discontinued abacavir for reasons unrelated to hypersensitivity (e.g., interruption in drug supply, discontinuance of abacavir during treatment for other medical conditions), some may have had symptoms present before discontinuance of the drug that were consistent with hypersensitivity but were attributed to other medical conditions (e.g., acute onset respiratory disease, gastroenteritis, adverse reactions to other drugs). Most of the hypersensitivity reactions reported following reintroduction of abacavir in these patients were indistinguishable from hypersensitivity reactions associated with abacavir rechallenge (i.e., short time to onset, increased severity of symptoms, poor outcome including death).Hypersensitivity reactions can occur within hours after abacavir is reintroduced; however, in some cases, these reactions occurred days to weeks following reintroduction of the drug. Lactic acidosis and severe hepatomegaly with steatosis (sometimes fatal) have been reported rarely in patients receiving abacavir and also have been reported in patients receiving dideoxynucleoside reverse transcriptase inhibitors. Most reported cases have involved women; obesity and long-term therapy with a nucleoside reverse transcriptase inhibitor also may be risk factors. Increased serum concentrations of Gamma-glutamyltransferase (GGT, GGPT) have been reported in patients receiving abacavir. Hypersensitivity reactions reported in patients receiving abacavir are characterized by the appearance of manifestations indicating involvement of multiple organ and body systems; these reactions have occurred in association with anaphylaxis, liver failure, renal failure, hypotension, and death. The most frequent manifestations of hypersensitivity reactions to abacavir include fever, rash, fatigue, GI symptoms such as nausea, vomiting, diarrhea, and abdominal pain, and respiratory symptoms such as pharyngitis, dyspnea, and cough. Other signs and symptoms include malaise, lethargy, myalgia, myolysis, headache, arthralgia, edema, paresthesia, lymphadenopathy, and mucous membrane lesions (e.g., conjunctivitis, mouth ulceration). Respiratory symptoms, including cough, dyspnea, and pharyngitis, have been reported in approximately 20% of patients with hypersensitivity reactions to abacavir. Fatalities have occurred in patients who developed hypersensitivity reactions in which the initial presentation included respiratory symptoms; some patients who experienced fatal hypersensitivity reactions were initially diagnosed as having an acute respiratory disease (pneumonia, bronchitis, flu-like illness). Hypersensitivity reactions can occur without rash; if rash occurs, it usually is maculopapular or urticarial, but may be variable in appearance. Laboratory abnormalities reported in patients experiencing a hypersensitivity reaction to abacavir include lymphopenia and increases in serum concentrations of liver enzymes, creatine kinase (CK, creatine phosphokinase, CPK), or creatinine. For more Drug Warnings (Complete) data for ABACAVIR SULFATE (17 total), please visit the HSDB record page. |
| 分子式 |
C28H38N12O6S
|
|---|---|
| 分子量 |
670.74312
|
| 精确质量 |
670.276
|
| CAS号 |
136777-48-5
|
| 相关CAS号 |
Abacavir;136470-78-5;Abacavir sulfate;188062-50-2;Abacavir monosulfate;216699-07-9
|
| PubChem CID |
441384
|
| 外观&性状 |
White to off-white solid
|
| LogP |
3.921
|
| tPSA |
286.74
|
| 氢键供体(HBD)数目 |
8
|
| 氢键受体(HBA)数目 |
16
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
47
|
| 分子复杂度/Complexity |
496
|
| 定义原子立体中心数目 |
4
|
| SMILES |
S(=O)(=O)(O)O.OC[C@@H]1C=C[C@@H](C1)N1C=NC2C1=NC(N)=NC=2NC1CC1.OC[C@@H]1C=C[C@@H](C1)N1C=NC2C1=NC(N)=NC=2NC1CC1
|
| InChi Key |
MCGSCOLBFJQGHM-SCZZXKLOSA-N
|
| InChi Code |
InChI=1S/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19)/t8-,10+/m1/s1
|
| 化学名 |
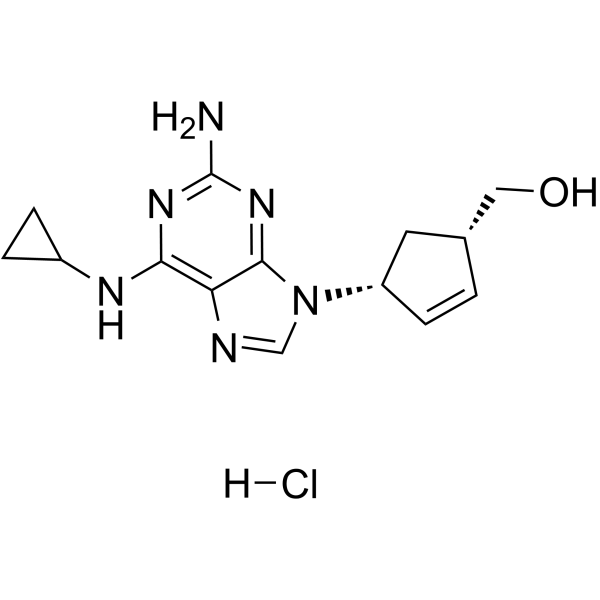
[(1S,4R)-4-[2-amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl]methanol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4909 mL | 7.4545 mL | 14.9089 mL | |
| 5 mM | 0.2982 mL | 1.4909 mL | 2.9818 mL | |
| 10 mM | 0.1491 mL | 0.7454 mL | 1.4909 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Mycophenolate Mofetil and Abacavir Treatment in HIV Patients With Failed Anti-HIV Treatment
CTID: NCT00021489
Phase: Phase 2 Status: Withdrawn
Date: 2021-11-01