| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|

| 靶点 |
DPP-4 (IC50 <10 nM)
Alogliptin benzoate (SYR-322) targets dipeptidyl peptidase 4 (DPP-4) (IC50 = 0.68 nM; Ki = 0.56 nM) [1] Alogliptin benzoate (SYR-322) shows high selectivity over other DPP family enzymes: DPP-8 (IC50 = 4100 nM), DPP-9 (IC50 = 7500 nM), FAP (IC50 > 10,000 nM) [1] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:阿格列汀(也称为 SYR-322)是一种新型、有效、口服生物可利用的选择性 DPP-4(丝氨酸蛋白酶二肽基肽酶 IV)抑制剂,IC50 值为 2.6 nM,其活性大于 10,000 倍。 DPP-4 的选择性高于密切相关的 DPP-8 和 DPP-9。是2010年在日本上市的抗糖尿病药; 2013 年,FDA 批准了该药物的三种剂型:单独使用的商品名为 Nesina,与二甲双胍联合使用的商品名为 Kazano,与吡格列酮联合使用的商品名为 Oseni。与其他治疗 2 型糖尿病的药物一样,阿格列汀不会降低心脏病发作和中风的风险。阿格列汀和其他格列汀通常与二甲双胍联合用于单独使用二甲双胍无法充分控制糖尿病的患者。激酶测定:苯甲酸阿格列汀 (SYR 322) 是一种有效的选择性 DPP-4 抑制剂,IC50 <10 nM,选择性比 DPP-8 和 DPP-9 高 10,000 倍以上。
它强效抑制重组人DPP-4酶活性,对DPP-8和DPP-9的选择性>6000倍,浓度高达10 μM时对FAP无显著抑制作用[1] - 在人血浆样本中,Alogliptin benzoate(0.1–10 nM)以剂量依赖性方式抑制内源性DPP-4活性,IC50为0.85 nM。它延长血浆中外源添加的GLP-1(7-36)酰胺的半衰期(10 nM时从1.8分钟延长至12.5分钟)[1] - 在大鼠胰岛细胞中,Alogliptin benzoate(1–100 nM)增强GLP-1诱导的胰岛素分泌(10 nM时增加2.3倍),不影响基础胰岛素释放。它还抑制胰岛细胞培养物中GLP-1的降解(10 nM时减少约78%)[2] - 浓度高达10 μM时,对人肝细胞、肾近端小管细胞或胰腺β细胞无细胞毒性(活力较对照组>90%)[2] |
| 体内研究 (In Vivo) |
阿格列汀(SYR-322)可产生剂量依赖性的葡萄糖耐量改善,并提高雌性 Wistar 脂肪大鼠的血浆胰岛素水平。急性给予阿格列汀会导致血浆 DPP-4 活性显着降低,并增加血浆活性 GLP-1。阿格列汀在 0.3 mg/kg 及更高剂量时可改善葡萄糖耐量,血浆 IRI 呈剂量依赖性增加,表明葡萄糖耐量改善是由于阿格列汀增强胰岛素分泌的能力所致。
在db/db小鼠(2型糖尿病模型)中:口服Alogliptin benzoate(1、3、10 mg/kg/天)持续28天,以剂量依赖性方式降低空腹血糖(FBG)和非空腹血糖(NFBG)。10 mg/kg剂量下,FBG较溶媒组降低约42%,糖化血红蛋白(HbA1c)降低约1.8%(从8.9%降至7.1%)[2] - 它改善db/db小鼠的葡萄糖耐量:口服葡萄糖耐量试验(OGTT)显示,10 mg/kg/天(28天)时葡萄糖曲线下面积(AUC)降低约35%。OGTT期间,血浆活性GLP-1水平增加约2.1倍,血浆胰岛素水平升高约1.5倍[2] - 在ZDF大鼠(2型糖尿病模型)中:口服Alogliptin benzoate(3 mg/kg/天)持续42天,FBG降低约38%,HbA1c降低约1.5%。胰腺组织中胰岛素含量增加约40%,表明胰腺β细胞功能改善[2] |
| 酶活实验 |
DPP-4检测:[1]
在二甲基亚砜(DMSO)中制备不同浓度(≤10mM终浓度)的试验化合物溶液,然后稀释到包含20mM Tris、pH 7.4的测定缓冲液中;20mM氯化钾;将人DPP-4(终浓度为0.1 nM)加入稀释液中,在环境温度下预孵育10分钟,然后用A-P-7-酰胺基-4-三氟甲基香豆素(AP-AFC;终浓度为10μM)引发反应。反应混合物的总体积为10-100μL,具体取决于所使用的测定格式(384或96孔板)。对反应进行动力学跟踪(激发λ=400 nm;发射λ=505 nm)5-10分钟,或在10分钟后测量终点。使用标准数学模型从酶进程曲线计算抑制常数(IC50)。[2] 肝微粒体稳定性:[1] 试验化合物(1μM)在37°C下在含有大鼠或人肝微粒体(1 mg/mL蛋白质)和NADPH(烟酰胺腺嘌呤二核苷酸磷酸盐,还原形式)(4 mM)的磷酸盐缓冲液(50 mM,pH 7.4)中孵育。在0、5、15、30分钟的时间过程中,用三氯乙酸(0.3M)淬灭孵育混合物。将淬火溶液离心,转移上清液进行LC/MS定量。试验化合物的半衰期由化合物随时间变化的稳定性曲线得出。[2] 阿格列汀(也称为 SYR-322)是一种新型、有效、选择性、口服生物可利用的 DPP-4(丝氨酸蛋白酶二肽基肽酶 IV)抑制剂。它的 IC50 值为 2.6 nM,对 DPP-4 的选择性比 DPP-8 和 DPP-9(两种密切相关的酶)高 10,000 倍以上。即使浓度高达 30 μM,阿格列汀也不会阻断 hERG 通道或抑制 CYP-450 酶活性。 Alogliptin Benzate(SYR 322) 是一种有效的选择性 DPP-4 抑制剂,IC50 小于 10 nM。其选择性比 DPP-8 和 DPP-9 高出 10,000 倍以上。 DPP-4酶活性实验:重组人DPP-4蛋白(5 nM)与荧光标记底物(Ala-Pro-AMC)、反应缓冲液(50 mM Tris-HCl pH 7.5、100 mM NaCl、1 mM EDTA)在37°C孵育30分钟。底物添加前10分钟加入浓度范围为0.01–100 nM的Alogliptin benzoate。荧光光谱法(激发光360 nm,发射光460 nm)检测AMC的释放量。相对于溶媒对照组计算抑制率,非线性回归确定IC50值[1] - DPP家族选择性实验:重组人DPP-8、DPP-9和FAP蛋白(各5 nM)分别与相应荧光底物、反应缓冲液在与DPP-4实验相同的条件下孵育。加入Alogliptin benzoate(0.1–10,000 nM),测量荧光强度以计算每种酶的IC50值[1] |
| 细胞实验 |
胰岛细胞胰岛素分泌实验:分离大鼠胰岛并培养24小时。胰岛用Alogliptin benzoate(1–100 nM)预处理1小时,再用GLP-1(7-36)酰胺(10 nM)+ 葡萄糖(16.7 mM)刺激2小时。ELISA量化培养上清液中的胰岛素。GLP-1降解实验中,胰岛与GLP-1 + Alogliptin benzoate孵育,不同时间点采集样本测量活性GLP-1水平[2]
- 血浆DPP-4抑制实验:人血浆与Alogliptin benzoate(0.1–10 nM)混合,37°C孵育15分钟。以Ala-Pro-AMC为底物测量DPP-4活性,检测荧光强度。GLP-1稳定性实验中,血浆中加入GLP-1(7-36)酰胺 + 药物,不同时间点采集样本测量活性GLP-1水平[1] |
| 动物实验 |
The N-STZ-1.5 rats
0.1, 0.3, 1 or 3 mg/kg p.o. Neonatally streptozotocin-induced diabetic rats (N-STZ-1.5 rats), a non-obese model of type 2 diabetes, were used in these studies. The effects of alogliptin on DPP-4 activity and glucagon-like peptide 1 (GLP-1) concentration were determined by measuring their levels in plasma. In addition, the effects of alogliptin on an oral glucose tolerance test were investigated by using an SU secondary failure model. Key findings: Alogliptin dose dependently suppressed plasma DPP-4 activity leading to an increase in the plasma active form of GLP-1 and improved glucose excursion in N-STZ-1.5 rats. Repeated administration of glibenclamide resulted in unresponsiveness or loss of glucose tolerance typical of secondary failure. In these rats, alogliptin exhibited significant improvement of glucose excursion with significant increase in insulin secretion. By contrast, glibenclamide and nateglinide had no effect on the glucose tolerance of these rats. Significance: The above findings suggest that alogliptin was effective at improving glucose tolerance and therefore overcoming SU induced secondary failure in N-STZ-1.5 rats.[2] db/db mouse type 2 diabetes model: 8-week-old male db/db mice were randomized into control (vehicle) and Alogliptin benzoate treatment groups (1, 3, 10 mg/kg/day, oral). Vehicle was 0.5% carboxymethylcellulose (CMC) + 0.1% Tween 80. Drugs were administered once daily for 28 days. Fasting blood glucose was measured weekly; HbA1c was measured at day 0 and day 28. OGTT was performed at day 21 (oral glucose load: 2 g/kg), and blood samples were collected to measure glucose, insulin, and active GLP-1 levels [2] - ZDF rat type 2 diabetes model: 10-week-old male ZDF rats were divided into control and treatment groups (3 mg/kg/day Alogliptin benzoate, oral). Drugs were administered once daily for 42 days. Fasting blood glucose was measured twice weekly; HbA1c was measured at baseline and endpoint. Pancreatic tissues were excised at euthanasia to quantify insulin content [2] - Pharmacokinetic study: Male Sprague-Dawley rats (250–300 g) and beagle dogs (8–10 kg) were administered Alogliptin benzoate via oral gavage (10 mg/kg) or intravenous injection (2 mg/kg). Blood samples were collected at 0, 0.5, 1, 2, 4, 8, 12, 24 hours post-administration. Plasma drug concentrations were measured by LC-MS/MS, and pharmacokinetic parameters were calculated using non-compartmental analysis [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption The pharmacokinetics of NESINA was also shown to be similar in healthy subjects and in patients with type 2 diabetes. When single, oral doses up to 800 mg in healthy subjects and type 2 diabetes patients are given, the peak plasma alogliptin concentration (median Tmax) occurred 1 to 2 hours after dosing. Accumulation of aloglipin is minimal. The absolute bioavailability of NESINA is approximately 100%. Food does not affect the absorption of alogliptin. Route of Elimination Renal excretion (76%) and feces (13%). 60% to 71% of the dose is excreted as unchanged drug in the urine. Volume of Distribution Following a single, 12.5 mg intravenous infusion of alogliptin to healthy subjects, the volume of distribution during the terminal phase was 417 L, indicating that the drug is well distributed into tissues. Clearance Renal clearance = 9.6 L/h (this value indicates some active renal tubular secretion); Systemic clearance = 14.0 L/h. The primary route of elimination of (14C) alogliptin-derived radioactivity occurs via renal excretion (76%) with 13% recovered in the feces, achieving a total recovery of 89% of the administered radioactive dose. The renal clearance of alogliptin (9.6 L/hr) indicates some active renal tubular secretion and systemic clearance was 14.0 L/hr. Alogliptin does not undergo extensive metabolism and 60% to 71% of the dose is excreted as unchanged drug in the urine. The absolute bioavailability of NESINA is approximately 100%. Administration of NESINA with a high-fat meal results in no significant change in total and peak exposure to alogliptin. NESINA may therefore be administered with or without food. Following a single, 12.5 mg intravenous infusion of alogliptin to healthy subjects, the volume of distribution during the terminal phase was 417 L, indicating that the drug is well distributed into tissues. Alogliptin is 20% bound to plasma proteins. View More
Metabolism / Metabolites
Biological Half-Life Terminal half-life = 21 hours At the maximum recommended clinical dose of 25 mg, Nesina was eliminated with a mean terminal half-life of approximately 21 hours. Oral bioavailability: 82% in rats, 79% in dogs [1] - Plasma half-life (t1/2): 6.8 hours in rats, 11.2 hours in dogs [1] - Plasma protein binding rate: 20% in human plasma, 18% in rat plasma, 22% in dog plasma (equilibrium dialysis assay) [1] - Tissue distribution: In rats, highest concentrations in kidney (2.1-fold vs. plasma), liver (1.8-fold vs. plasma), and small intestine (1.5-fold vs. plasma); minimal penetration into the central nervous system (<0.5% of plasma concentration) [1] - Metabolism: Minimally metabolized in liver (only ~10% of dose metabolized); major metabolite is inactive [1] - Excretion: 70% excreted unchanged in urine, 20% in feces within 72 hours post-administration in rats [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary\n
\nIDENTIFICATION AND USE: Alogliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus; but not for treatment of type 1 diabetes or diabetic ketoacidosis. HUMAN EXPOSURE AND TOXICITY: During clinical trials patients receiving alogliptin 25 mg daily reported adverse reactions including pancreatitis (0.2%), hypersensitivity reactions (0.6%), a single event of serum sickness, nasopharyngitis (4.4%), hypoglycemia (1.5%), headache (4.2%) and upper respiratory tract infection (4.2%). In elderly patients the incidence of hypoglycemia with alogliptin increased to 5.4%. Postmarketing, patients taking alogliptin reported acute pancreatitis and serious hypersensitivity reactions. These reactions include anaphylaxis, angioedema and severe cutaneous adverse reactions, including Stevens-Johnson syndrome. There have been postmarketing reports of fatal and nonfatal hepatic failure in patients taking Nesina. ANIMAL STUDIES: In a fertility study in rats, alogliptin had no adverse effects on early embryonic development, mating or fertility at doses up to 500 mg/kg, or approximately 172 times the clinical dose based on plasma drug exposure (AUC). Alogliptin administered to pregnant rabbits and rats during the period of organogenesis was not teratogenic at doses of up to 200 mg/kg and 500 mg/kg, or 149 times and 180 times, respectively, the clinical dose based on plasma drug exposure (AUC). Doses of alogliptin up to 250 mg/kg (approximately 95 times clinical exposure based on AUC) given to pregnant rats from gestation Day 6 to lactation Day 20 did not harm the developing embryo or adversely affect growth and development of offspring. Placental transfer of alogliptin into the fetus was observed following oral dosing to pregnant rats. Alogliptin is secreted in the milk of lactating rats in a 2:1 ratio to plasma. No drug-related tumors were observed in mice after administration of 50, 150 or 300 mg/kg alogliptin for two years, or up to approximately 51 times the maximum recommended clinical dose of 25 mg, based on AUC exposure. Alogliptin was not mutagenic or clastogenic, with and without metabolic activation, in the Ames test with S. typhimurium and E. coli or the cytogenetic assay in mouse lymphoma cells. Alogliptin was negative in the in vivo mouse micronucleus study.\n \n\nHepatotoxicity\n \nLiver injury due to alogliptin is rare. In large clinical trials, serum enzyme elevations were uncommon (1% to 3%) and no greater than with comparator arms or placebo. In these studies, no instances of clinically apparent liver injury with jaundice were reported. Since licensure, instances of serum enzyme elevations and acute hepatitis including acute liver failure attributed to alogliptin have been reported to the FDA and the sponsor. These cases have not been reported in the literature and the clinical features have not been defined. Cases of clinically apparent acute liver injury have been reported with other DPP-4 inhibitors such as sitagliptin and saxagliptin. The latency to onset was typically within 2 to 12 weeks of starting and the pattern of liver enzyme elevations was usually hepatocellular. Immunoallergic features were often present. Most cases were self-limited in course and rapidly reversed once the medication was stopped.\n \nLikelihood score: E* (unproven but suspected cause of acute, idiosyncratic liver injury).\n\n \n\n\n\n \n \n\nView More\n\nEffects During Pregnancy and Lactation\n \nInteractions\n \nWhen alogliptin is used in combination with an insulin secretagogue (e.g., a sulfonylurea) or insulin, the incidence of hypoglycemia is increased compared with sulfonylurea or insulin monotherapy. Therefore, patients receiving alogliptin may require a reduced dosage of the concomitant insulin secretagogue or insulin to reduce the risk of hypoglycemia.\n \nProtein Binding\n \nAlogliptin is 20% bound to plasma proteins.\n\n In vitro toxicity: Alogliptin benzoate at concentrations up to 10 μM shows no significant cytotoxicity to human hepatocytes (HepG2), renal proximal tubule cells (HK-2), or pancreatic β-cells (INS-1) [2] - Acute toxicity: LD50 > 2000 mg/kg in rats and mice (oral administration); no mortality or severe toxic symptoms (lethargy, gastrointestinal distress) observed at doses up to 2000 mg/kg [2] - Repeat-dose toxicity: In a 90-day study in rats (oral doses of 10, 30, 100 mg/kg/day), the drug was well-tolerated. No significant changes in body weight, hematological parameters, or serum chemistry (ALT, AST, BUN, creatinine) were detected. Histological examination of liver, kidney, pancreas, and heart revealed no abnormal lesions [2] - Drug-drug interaction potential: Does not inhibit or induce major CYP450 enzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4) at therapeutic concentrations [1] |
| 参考文献 | |
| 其他信息 |
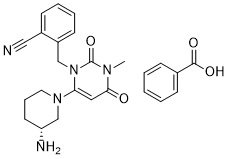
Alogliptin benzoate is a benzoate salt obtained by combining equimolar amounts of alogliptin and benzoic acid. Used for treatment of type 2 diabetes. It has a role as an EC 3.4.14.5 (dipeptidyl-peptidase IV) inhibitor and a hypoglycemic agent. It contains an alogliptin(1+).
Alogliptin Benzoate is the benzoate salt form of alogliptin, a selective, orally bioavailable, pyrimidinedione-based inhibitor of dipeptidyl peptidase 4 (DPP-4), with hypoglycemic activity. In addition to its effect on glucose levels, alogliptin may inhibit inflammatory responses by preventing the toll-like receptor 4 (TLR-4)-mediated formation of proinflammatory cytokines. See also: Alogliptin (has active moiety); Alogliptin Benzoate; Pioglitazone Hydrochloride (component of); Alogliptin Benzoate; METformin Hydrochloride (component of). Drug Indication Vipidia is indicated in adults aged 18 years and older with type 2 diabetes mellitus to improve glycaemic control in combination with other glucose lowering medicinal products including insulin, when these, together with diet and exercise, do not provide adequate glycaemic control (see sections 4. 4, 4. 5 and 5. 1 for available data on different combinations). Alogliptin benzoate (SYR-322) is a potent, orally bioavailable, and highly selective dipeptidyl peptidase 4 (DPP-4) inhibitor [1,2] - Its mechanism of action involves reversible inhibition of DPP-4, which degrades incretin hormones (GLP-1 and GIP). This prolongs the half-life of GLP-1 and GIP, enhancing glucose-dependent insulin secretion and suppressing glucagon release, thereby reducing blood glucose levels [1,2] - It is indicated for the treatment of type 2 diabetes mellitus, as it improves glycemic control without causing hypoglycemia (in preclinical models) [2] - Favorable pharmacokinetic profile (long half-life, high oral bioavailability, minimal metabolism) supports once-daily oral administration [1] - Low plasma protein binding and minimal drug-drug interaction potential make it suitable for combination with other antidiabetic agents [1] |
| 分子式 |
C25H27N5O4
|
|---|---|
| 分子量 |
461.51
|
| 精确质量 |
461.206
|
| 元素分析 |
C, 65.06; H, 5.90; N, 15.17; O, 13.87
|
| CAS号 |
850649-62-6
|
| 相关CAS号 |
Alogliptin;850649-61-5;Alogliptin-13C,d3 benzoate; Alogliptin Benzoate;850649-62-6;Alogliptin-d3;1133421-35-8;Alogliptin-13C,d3 benzoate;Alogliptin-13C,d3;1246817-18-4
|
| PubChem CID |
16088021
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
671.2ºC at 760 mmHg
|
| 闪点 |
359.7ºC
|
| LogP |
2.544
|
| tPSA |
134.35
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
726
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C(C1C=CC=CC=1)(=O)O.C(C1C=CC=CC=1C#N)N1C(=O)N(C)C(=O)C=C1N1CCC[C@@H](N)C1
|
| InChi Key |
KEJICOXJTRHYAK-XFULWGLBSA-N
|
| InChi Code |
InChI=1S/C18H21N5O2.C7H6O2/c1-21-17(24)9-16(22-8-4-7-15(20)12-22)23(18(21)25)11-14-6-3-2-5-13(14)10-19;8-7(9)6-4-2-1-3-5-6/h2-3,5-6,9,15H,4,7-8,11-12,20H2,1H3;1-5H,(H,8,9)/t15-;/m1./s1
|
| 化学名 |
2-[[6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxopyrimidin-1-yl]methyl]benzonitrile;benzoic acid
|
| 别名 |
SYR-322 benzoate; SYR322; SYR 322; Alogliptin benzoate; Nesina; Kazano; Oseni
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.71 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.25 mg/mL (2.71 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 12.5 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.25 mg/mL (2.71 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 0.5% methylcellulose: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1668 mL | 10.8340 mL | 21.6680 mL | |
| 5 mM | 0.4334 mL | 2.1668 mL | 4.3336 mL | |
| 10 mM | 0.2167 mL | 1.0834 mL | 2.1668 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02756832 | Completed | Drug: Alogliptin Benzoate | Diabetes Mellitus | Takeda | September 20, 2016 | |
| NCT04980040 | Completed | Drug: Alogliptin Benzoate | Type 2 Diabetes Mellitus | Takeda | April 19, 2014 | |
| NCT02856113 | Completed | Drug: Alogliptin Benzoate Drug: Placebo |
Diabetes Mellitus, Type 2 | Takeda | October 14, 2016 | Phase 3 |
| NCT01990300 | Completed | Drug: Alogliptin/Pioglitazone | Type 2 Diabetes Mellitus | Takeda | November 28, 2011 | |
| NCT02798172 | Completed | Drug: Alogliptin and Metformin | Diabetes Mellitus, Type 2 | Fourth People's Hospital of Shenyang |
May 2014 | Not Applicable |
 |