| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Ornithine decarboxylase
|
|---|---|
| 体外研究 (In Vitro) |
依氟鸟氨酸是鸟氨酸脱羧酶的一种特异性、不可逆的抑制剂,被认为通过抑制毛囊中的这种酶来减缓头发生长[2]。
采用Franz扩散池对依氟鸟氨酸进行体外渗透研究。当将依氟鸟氨酸乳膏涂抹在用微针预处理过的小鼠皮肤区域时,依氟鸟氨酸的毛发生长抑制活性显著增强,很可能是因为微针产生的微孔允许依氟鸟氨酸渗透到皮肤中,这在体外渗透研究中得到了证实。免疫组织化学数据显示,将依氟鸟氨酸乳膏涂抹在微针预处理的皮肤区域,皮肤和毛囊的细胞增殖也明显受到抑制。[3] |
| 体内研究 (In Vivo) |
过去五十年来唯一被批准用于治疗人类非洲锥虫病的新药是依氟鸟氨酸。它主要用作对 melarsoprol 没有反应的布氏冈比亚锥虫感染的备用药物 [1]。当谈到减少毛发过多的参与者面部毛发的生长时,15% 依氟鸟氨酸霜的效果优于安慰剂。经过 24 周的治疗疗程后,58% 的依氟鸟氨酸患者和 34% 的安慰剂受试者的面部多毛症至少有所改善 [2]。当将依氟鸟氨酸乳膏施用到预先经过微针处理的小鼠皮肤区域时,依氟鸟氨酸的毛发生长抑制活性显着增加[3]。高血压14天后,依氟鸟氨酸治疗缩窄性高血压大鼠导致KCI和去甲肾上腺素收缩强度正常化以及乙酰胆碱松弛[4]。
|
| 酶活实验 |
In vitro permeation of eflornithine hydrochloride through mouse skin/盐酸依氟鸟氨酸体外经小鼠皮肤渗透研究[3]
如前所述,使用Franz扩散池仪完成体外渗透测定(Kumar等人,2012;Kumar et al. 2011;Naguib, Kumar, & Cui 2014)使用C57BL/6小鼠的下背皮肤。收集皮肤前24小时用电动剪发器修剪毛发。采集皮肤,用铝箔包裹,在- 20°C下保存最多一个月,并在需要时使用。在- 20°C下将皮肤冷冻(不使用冷冻保护剂)在文献中是常用的方法,并且这种皮肤样本经常用于渗透性研究(Stahl, Wohlert, & Kietzmann 2012)。Dennerlein等人表明,将新鲜切除的人体皮肤在- 20°C下冷冻和储存长达30天不会影响皮肤的渗透性(Dennerlein等人,2013年)。其他研究人员表明,将人体皮肤包裹在铝箔中并保存在- 26°C时,皮肤可保持其屏障性能长达6个月(Badran, Kuntsche, & Fahr 2009)。去除脂肪层后,将皮肤背朝上置于Franz扩散细胞上。接收器室装有5ml水,用Haake SC 100水循环器(ThermoScientific, Wellington, NH)保持在37°C。如前所述,在将毛发修饰的皮肤安装到Franz扩散池上之前,使用Dermaroller®微针辊进行处理(Kumar等人,2011;Naguib, Kumar, & Cui 2014)。将皮肤样品置于天平的平面上,微针滚轮在皮肤表面沿四个垂直方向滚动,每个方向滚动5次,共滚动20次,施加的压力为350 - 400g,在滚动滚轮的同时使用天平不断测量。皮肤扩散面积为0.64 cm2。供体室以500 μl的水注入盐酸依氟鸟氨酸4 mg,并用副膜覆盖以防止蒸发。在0、1、3、6、8和24 h后,从接收室中取出样品(150 μl),并立即补充新鲜培养基。使用高效液相色谱法对样品进行分析,方法采用先前描述的方法,并进行了修改(Saravanan et al. 2009)。色谱分析采用Agilent 1260 Infinity高效液相色谱仪,配备ZORBAX Eclipse Plus C18 (5 μm, 4.6 × 150 mm)色谱柱,流动相为乙腈-缓冲液(70%:30%,v/v)。将0.68 g磷酸一碱钾溶于1l水中制备缓冲液。流速0.8 ml/min。探测器波长为210 nm。 |
| 细胞实验 |
皮肤组织用福尔马林(10%)缓冲溶液固定24 h,用0.1 M磷酸钠缓冲液(pH 7.4)洗涤,用分级乙醇脱水,石蜡包埋,垂直切片。切片使用苏木精-伊红(H&E)或抗5-溴-2 ' -脱氧尿苷(BrdU)抗体在德克萨斯大学奥斯汀分校戴尔儿科研究所的组织学和组织处理设施进行染色。在安乐死前30分钟,以100 μg/g体重的剂量,在磷酸盐缓冲盐水(PBS, pH 7.4, 10 mM)中腹腔注射BrdU。所有皮肤切片均在Olympus BX53显微镜下检查[3]。
|
| 动物实验 |
In vivo efficacy study was performed in a mouse model by monitoring the re-growth of hair in the lower dorsal skin of mice after the eflornithine cream was applied onto an area pretreated with microneedles. The skin and the hair follicles in the treated area were also examined histologically[3].
Female C57BL/6 mice (8–10 weeks old) were are ideal for examining the physiological actions during different hair cycle phases due to the occurrence of naturally synchronized hair cycles with cyclic pigmentation (Slominski, Paus, & Costantino 1991). Each experimental group was composed of 3–4 mice. Hair in the lower dorsal skin of anesthetized mice was either trimmed using an electric clipper, plucked using GiGi® Honee warm wax as previously described (Xiao et al. 2012), or chemically removed using Nair® lotion. The skin area where the hair was removed was then treated with the eflornithine hydrochloride 13.9% cream (~50 mg per mouse per treatment) using a spatula two times a day in an interval of at least 8 h for a maximum period of 36 days. A group of mice whose hair in the application site was trimmed using a clipper were also treated with the microneedle roller every time before the application of eflornithine cream as previously described (Kumar et al. 2012). Briefly, mice were placed onto the flat surface of a balance, and the microneedle roller was rolled over the marked skin surface, 10 times parallel to mouse length, with an applying pressure of 350–400 g as indicated on the balance. In control groups, the hair in mouse dorsal skin was removed by trimming, plucking, or chemical depilation with Nair®, but the area was not treated with the eflornithine cream. The hair re-growth was evaluated by taking digital photographs of the mouse skin areas for a maximum period of 36 days after the first application of the eflornithine cream. On the last day of the study, animals were euthanized, and skin samples were collected from the treated areas for immunohistochemical studies.[3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administrations of eflornithine, peak plasma concentrations of eflornithine (Cmax) were achieved (Tmax) 3.5 hours post-dosing. The Cmax and AUC (area under the concentration-time curve) of eflornithine were not affected by food (high fat and high calories). Administration of crushed tablets in a standard pudding admixture had no effect on eflornithine exposure (Cmax and AUC6h). The mean percutaneous absorption of eflornithine in women with unwanted facial hair, from a 13.9% w/w cream formulation, is < 1% of the radioactive dose, following either single or multiple doses under conditions of clinical use, that included shaving within 2 hours before radiolabeled dose application in addition to other forms of cutting or plucking and tweezing to remove facial hair. Steady state was reached within four days of twice-daily application. Following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day; 139 mg as anhydrous eflornithine hydrochloride), under conditions of clinical use in women with unwanted facial hair (n=10), the steady-state Cmax, Ctrough and AUC12hr were approximately 10 ng/mL, 5 ng/mL, and 92 ng hr/mL, respectively, expressed in terms of the anhydrous free base of eflornithine hydrochloride. At steady state, the dose-normalized peak concentrations (Cmax) and the extent of daily systemic exposure (AUC) of eflornithine following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day) is estimated to be approximately 100- and 60-fold lower, respectively, when compared to 370 mg/day once-daily oral doses. This compound is not known to be metabolized and is primarily excreted unchanged in the urine. Eflornithine volume of distribution (Vz/F) is 24.3 L. The clearance (CL/F) of eflornithine is 5.3 L/h. The mean percutaneous absorption of eflornithine in women with unwanted facial hair, from a 13.9% w/w cream formulation, is < 1% of the radioactive dose, following either single or multiple doses under conditions of clinical use, that included shaving within 2 hr before radiolabeled dose application in addition to other forms of cutting or plucking and tweezing to remove facial hair. Following twice daily application of 0.5 g of the cream (total dose 1.0 g/day; 139 mg as anhydrous eflornithine hydrochloride), under conditions of clinical use in women with unwanted facial hair (n=10), the steady-state Cmax, Ctrough and AUC12hr were approximately 10 ng/mL, 5 ng/mL, and 92 nghr/mL, respectively, expressed in terms of the anhydrous free base of eflornithine hydrochloride. At steady state, the dose-normalized peak concentrations (Cmax) and the extent of daily systemic exposure (AUC) of eflornithine following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day) is estimated to be approximately 100- and 60-fold lower, respectively, when compared to 370 mg/day once-daily oral doses. Eflornithine is not metabolized and is excreted unchanged in urine. For more Absorption, Distribution and Excretion (Complete) data for Eflornithine (8 total), please visit the HSDB record page. Metabolism / Metabolites This compound is not known to be metabolized and is primarily excreted unchanged in the urine. Biological Half-Life The terminal plasma elimination half-life of eflornithine was 3.5 hours, and the apparent steady-state plasma half-life of eflornithine was approximately 8 hours. The apparent steady-state plasma t1/2 of eflornithine was approximately 8 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Maternal intravenous eflornithine 400 mg/kg daily for 7 days did not cause any adverse serious effects in breastfed infants. After topical application, eflornithine is poorly absorbed so it is not likely to reach the bloodstream of the infant or cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants A cohort of 33 infants who were breastfed (extent not stated) by hospitalized mothers taking nifurtimox was followed in the Democratic Republic of the Congo. Thirty mothers took a full course of 30 doses of oral nifurtimox 15 mg/kg daily and all received 14 doses of intravenous eflornithine 400 mg/kg daily for 7 days for human African trypanosomiasis. (sleeping sickness). Nursing mothers also took a median of 4 other concomitant medications, including amoxicillin, ciprofloxacin, metronidazole, trimethoprim-sulfamethoxazole, aspirin, and diclofenac (1 patient each); hydrocortisone, promethazine and quinine (2 patients each); levamisole (6 patients); sulfadoxine-pyrimethamine (8 patients); dipyrone (13 patients); acetaminophen (16 patients); and mebendazole (17 patients). No serious adverse events were reported in any of the breastfed infants. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 | |
| 其他信息 |
Eflornithine hydrochloride monohydrate is the hydrochloride and hydrate of the trypanocidal drug eflornithine. It is a hydrochloride and a hydrate. It contains an eflornithine.
Eflornithine Hydrochloride is the hydrochloride form of eflornithine, a difluoromethylated ornithine compound with antineoplastic activity. Eflornithine irreversibly inhibits ornithine decarboxylase, an enzyme required for polyamine biosynthesis, thereby inhibiting the formation and proliferation of tumor cells. Polyamines are involved in nucleosome oligomerization and DNA conformation, creating a chromatin environment that stimulates neoplastic transformation of cells. (NCI04) An inhibitor of ornithine decarboxylase, the rate limiting enzyme of the polyamine biosynthetic pathway. See also: Eflornithine (has active moiety). Drug Indication Treatment of facial hirsutism in women. Eflornithine is the only new molecule registered for the treatment of human African trypanosomiasis over the last 50 years. It is the drug used mainly as a back-up for melarsoprol refractory Trypanosoma brucei gambiense cases. The most commonly used dosage regimen for the treatment of T. b. gambiensesleeping sickness consists of 100 mg kg(-1) body weight at intervals of 6 h for 14 days (150 mg kg(-1) body weight in children) of eflornithine given as short infusions. Its efficacy against Trypanosoma brucei rhodesiense is limited due to the innate lack of susceptibility of this parasite based on a higher ornithine decarboxylase turnover. Adverse drug reactions during eflornithine therapy are frequent and the characteristics are similar to other cytotoxic drugs for the treatment of cancer. Their occurrence and intensity increase with the duration of treatment and the severity of the general condition of the patient. Generally, adverse reactions to eflornithine are reversible after the end of treatment. They include convulsions (7%), gastrointestinal symptoms like nausea, vomiting and diarrhea (10%-39%), bone marrow toxicity leading to anemia, leucopenia and thrombocytopenia (25-50%), hearing impairment (5% in cancer patients) and alopecia (5-10%). The drug arrests embryonic development in mice, rats and rabbits but the extent of excretion into breast milk is unknown. The mean half-life is around 3-4 h and the volume of distribution in the range of 0.35 l kg(-1). Renal clearance is about 2 ml min kg(-1) (i.v.) and accounts for more than 80% of drug elimination. Bioavailability of an orally administered 10 mg kg(-1) dose was estimated at 54%. One of the major determinants of successful treatment seems to be the cerebrospinal fluid drug level reached during treatment, and it was shown that levels above 50 micro mol l(-1) must be reached to attain the consistent clearance of parasites. Based on its trypanostatic rather than trypanocidal mode of action, it is a rather slow-acting drug.[1] Eflornithine is a specific, irreversible inhibitor of the enzyme ornithine decarboxylase which is thought to slow hair growth by inhibiting this enzyme in hair follicles. Percutaneous absorption of eflornithine in women with unwanted facial hair (hirsutism) was < 1% when the 15% cream was applied twice daily to a shaved 50 cm2 area of skin under the chin. In clinical studies in women with excessive, unwanted facial hair, eflornithine 15% cream was superior to placebo in reducing hair growth, as demonstrated by objective and subjective methods, after 2 to 8 weeks' treatment. After 24 weeks' treatment, 58% of eflornithine and 34% of placebo recipients had at least some improvement in facial hirsutism (for the purposes of this analysis all patients not assessed at week 24 were considered to be worse or to have no improvement). In addition, 32 versus 8% of patients were judged to be successfully treated (at least marked improvement). Hair growth returned to pretreatment rates within 8 weeks of stopping treatment. Use of a self-assessment questionnaire to assess the effect of study treatment on 6 aspects of patient well-being showed that eflornithine reduced the mean level of overall discomfort and bother by 33 versus 15% in placebo recipients. Adverse events mostly affected the skin. Only burning/stinging/tingling was markedly more common with eflornithine than with placebo.[2] Context: Facial hirsutism is a cosmetic concern for women and can lead to significant anxiety and lack of self-esteem. Eflornithine cream is indicated for the treatment of facial hirsutism. However, limited success rate and overall patient's satisfaction, even with a long-term and high-frequency application, leave room for improvement. Objective: The objective of this study is to test the effect of microneedle treatment on the in vitro skin permeation and the in vivo efficacy of eflornithine cream in a mouse model. Materials and method: In vitro permeation study of eflornithine was performed using Franz diffusion cell. In vivo efficacy study was performed in a mouse model by monitoring the re-growth of hair in the lower dorsal skin of mice after the eflornithine cream was applied onto an area pretreated with microneedles. The skin and the hair follicles in the treated area were also examined histologically. Results and discussion: The hair growth inhibitory activity of eflornithine was significantly enhanced when the eflornithine cream was applied onto a mouse skin area pretreated with microneedles, most likely because the micropores created by microneedles allowed the permeation of eflornithine into the skin, as confirmed in an in vitro permeation study. Immunohistochemistry data revealed that cell proliferation in the skin and hair follicles was also significantly inhibited when the eflornithine cream was applied onto a skin area pretreated with microneedles. Conclusion: The integration of microneedle treatment into topical eflornithine therapy represents a potentially viable approach to increase eflornithine's ability to inhibit hair growth. Keywords: Cell proliferation; hair growth inhibition; microneedles; skin permeation; unwanted hair growth.[3] This study examined the temporal effects of the polyamine synthesis inhibitor eflornithine (alpha-difluoromethylornithine) on vascular responses to KCI, norepinephrine, sodium nitroprusside and acetylcholine in aortic rings from coarctation hypertensive rats. Coarctation hypertension reduced the contractile response of aortic rings to KCI and norepinephrine, increased sensitivity (reduced the EC50 value) to norepinephrine and attenuated relaxation to acetylcholine by 14 days of hypertension. Treatment of coarctation hypertensive rats with eflornithine resulted in a normalization of the contractile intensity to KCI and norepinephrine and relaxations to acetylcholine by 14 days of hypertension. Responses to sodium nitroprusside were similar in all groups at all time points. Hyperresponsiveness to norepinephrine produced by coarctation of the aorta was not affected by eflornithine. These studies indicate that normalization of vascular function can occur in the presence of significantly elevated blood pressure upon chronic administration of eflornithine. This functional normalization correlates with eflornithine-mediated regression of structural abnormalities normally associated with pressure overload hypertension.[4] |
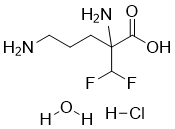
| 分子式 |
C6H15CLF2N2O3
|
|---|---|
| 分子量 |
236.6447
|
| 精确质量 |
236.074
|
| 元素分析 |
C, 30.45; H, 6.39; Cl, 14.98; F, 16.06; N, 11.84; O, 20.28
|
| CAS号 |
96020-91-6
|
| 相关CAS号 |
Eflornithine;70052-12-9;L-Eflornithine monohydrochloride;69955-42-6;Eflornithine hydrochloride;68278-23-9;L-Eflornithine;66640-93-5; 96020-91-6 (HCl hydrate) 68278-23-9 (HCl); 70050-55-4 (R-isomer); 69955-42-6 (S-isomer); 66640-93-5 (L-isomer)
|
| PubChem CID |
441361
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.293g/cm3
|
| 沸点 |
347ºC at 760mmHg
|
| 熔点 |
>210ºC (dec.)
|
| 闪点 |
163.7ºC
|
| LogP |
1.91
|
| tPSA |
98.57
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
166
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.O=C(C(C(F)F)(CCCN)N)O.O
|
| InChi Key |
FJPAMFNRCFEGSD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H12F2N2O2.ClH.H2O/c7-4(8)6(10,5(11)12)2-1-3-9/h4H,1-3,9-10H2,(H,11,12)1H1H2
|
| 化学名 |
2,5-diamino-2-(difluoromethyl)pentanoic acid hydrochloride hydrate
|
| 别名 |
Ornidyl; CPP-1X; RMI71782; Eflornithine hydrochloride hydrate; Eflornithine hydrochloride monohydrate; Vaniqa; Eflornithine HCl; dfmo; MDL 71,782 A; Eflornithine HCl hydrate; RMI-71782; RMI 71782;
DL-Ornithine hydrochloride;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~83.33 mg/mL (~352.14 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (422.58 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.2258 mL | 21.1291 mL | 42.2583 mL | |
| 5 mM | 0.8452 mL | 4.2258 mL | 8.4517 mL | |
| 10 mM | 0.4226 mL | 2.1129 mL | 4.2258 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。