| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
Ornithine decarboxylase
|
|---|---|
| 体外研究 (In Vitro) |
Eflornithine (D/L-DFMO) 是 ODC 的抑制剂,ODC 是真核多胺生产中的第一种酶。依氟鸟氨酸 (DFMO) 的两种对映体都会不可逆地灭活 ODC。两种依氟鸟氨酸对映体(L-依氟鸟氨酸和 D-依氟鸟氨酸)以时间和浓度依赖性方式降低 ODC 活性。产生酶-抑制剂复合物的D-依氟鸟氨酸、L-依氟鸟氨酸和依氟鸟氨酸的抑制剂解离常数(KD)值分别为28.3±3.4、1.3±0.3和2.2±。 0.4 µM。 D-依氟鸟氨酸、L-依氟鸟氨酸和依氟鸟氨酸不可逆相的抑制剂失活常数 (Kinact) 分别为 0.25±0.03、0.15±0.03 和 0.15±0.03 min-1。用 L-依氟鸟氨酸或 D-依氟鸟氨酸处理人结肠肿瘤来源的 HCT116 细胞,会以浓度依赖性方式降低细胞多胺水平 [1]。对映异构体在体外表现出不同的效力,L-对映异构体对靶酶鸟氨酸脱羧酶的亲和力高出 20 倍 [2]。 L-eflornithine 似乎对培养的冈比亚拟杆菌寄生虫也更有效 [2]。
|
| 体内研究 (In Vivo) |
与 D-依氟鸟氨酸相比,效力更强的 L-依氟鸟氨酸在血浆和脑脊液 (CSF) 中的含量显着降低。 L-依氟鸟氨酸的典型血浆浓度是 D-对映体浓度的 52%。 L-依氟鸟氨酸和 D-依氟鸟氨酸的典型口服清除率分别为 17.4 L/h 和 8.23 L/h [2]。
|
| 酶活实验 |
外消旋二氟甲基鸟氨酸(D/L-DFMO)是ODC(鸟氨酸脱羧酶)的抑制剂,ODC是真核多胺生物合成中的第一种酶。D/L-DFMO是一种有效的抗寄生虫剂和哺乳动物细胞生长发育抑制剂。纯化的人odc催化的鸟氨酸脱羧具有高度的立体特异性。然而,两种DFMO对映体都以时间和浓度依赖的方式抑制ODC活性。在L-或D-DFMO治疗和透析去除游离抑制剂后,ODC活性未能恢复。D-、L-和D/L- dfmo形成酶-抑制剂复合物的抑制剂解离常数(K(D))分别为28.3+/-3.4、1.3+/-0.3和2.2+/-0.4微米。K(D)值差异均有统计学意义(P <0.05)。D-、L-和D/L- dfmo不可逆步骤的抑制剂失活常数K(inact)分别为0.25+/-0.03、0.15+/-0.03和0.15+/-0.03 min(-1)。后两者差异无统计学意义(P < 0.01)。与d -鸟氨酸(IC50约为1.5 mM)相比,D-DFMO是一种更有效的odc催化l -鸟氨酸脱羧抑制剂(IC50约为7.5微米)。用L-或D-DFMO处理人结肠肿瘤来源的HCT116细胞,以浓度依赖的方式降低细胞多胺含量。这些结果表明,DFMO的两个对映体不可逆地失活ODC,并表明这种失活是通过一个共同的机制发生的。这两种对映体都与ODC形成酶抑制剂复合物,但与D-DFMO相比,L-DFMO形成这些复合物的可能性是D-DFMO的20倍。ODC失活的不可逆反应速率对于L-和d -对映体是相似的。DFMO对映体之间的这种意想不到的相似性,与底物鸟氨酸的高度立体特异性相反,似乎是由于抑制剂的α取代基。与L-或D/L- dfmo相比,D-对映体在某些临床应用中可能具有优势,例如降低正常组织毒性。[1]
|
| 动物实验 |
This study aimed to characterize the stereoselective pharmacokinetics of oral eflornithine in 25 patients with late-stage Trypanosoma brucei gambiense sleeping sickness. A secondary aim was to determine the concentrations of L- and D-eflornithine required in plasma or cerebrospinal fluid (CSF) for an efficient eradication of the T. brucei gambiense parasites. Patients were randomly allocated to receive either 100 (group I, n=12) or 125 (group II, n=13) mg/kg of body weight of drug every 6 h for 14 days. The concentrations of L- and D-eflornithine in the plasma and CSF samples were measured using a stereospecific liquid chromatographic method. Nonlinear mixed-effects modeling was used to characterize the plasma pharmacokinetics. The plasma concentrations of L-eflornithine were on average 52% (95% confidence interval [CI], 51, 54%; n=321) of the D-enantiomer concentrations. The typical oral clearances of L- and D-eflornithine were 17.4 (95% CI, 15.5, 19.3) and 8.23 (95% CI, 7.36, 9.10) liters/h, respectively. These differences were likely due to stereoselective intestinal absorption. The distributions of eflornithine enantiomers to the CSF were not stereoselective. A correlation was found between the probability of cure and plasma drug exposure, although it was not more pronounced for the L-enantiomer than for that of total eflornithine. This study may explain why oral treatment for late-stage human African trypanosomiasis (HAT) patients with racemic eflornithine has previously failed; the more potent L-enantiomer is present at much lower concentrations in both plasma and CSF than those of the D-enantiomer. Eflornithine stereoselective pharmacokinetics needs to be considered if an oral dosage regimen is to be explored further.[2]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administrations of eflornithine, peak plasma concentrations of eflornithine (Cmax) were achieved (Tmax) 3.5 hours post-dosing. The Cmax and AUC (area under the concentration-time curve) of eflornithine were not affected by food (high fat and high calories). Administration of crushed tablets in a standard pudding admixture had no effect on eflornithine exposure (Cmax and AUC6h). The mean percutaneous absorption of eflornithine in women with unwanted facial hair, from a 13.9% w/w cream formulation, is < 1% of the radioactive dose, following either single or multiple doses under conditions of clinical use, that included shaving within 2 hours before radiolabeled dose application in addition to other forms of cutting or plucking and tweezing to remove facial hair. Steady state was reached within four days of twice-daily application. Following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day; 139 mg as anhydrous eflornithine hydrochloride), under conditions of clinical use in women with unwanted facial hair (n=10), the steady-state Cmax, Ctrough and AUC12hr were approximately 10 ng/mL, 5 ng/mL, and 92 ng hr/mL, respectively, expressed in terms of the anhydrous free base of eflornithine hydrochloride. At steady state, the dose-normalized peak concentrations (Cmax) and the extent of daily systemic exposure (AUC) of eflornithine following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day) is estimated to be approximately 100- and 60-fold lower, respectively, when compared to 370 mg/day once-daily oral doses. This compound is not known to be metabolized and is primarily excreted unchanged in the urine. Eflornithine volume of distribution (Vz/F) is 24.3 L. The clearance (CL/F) of eflornithine is 5.3 L/h. The mean percutaneous absorption of eflornithine in women with unwanted facial hair, from a 13.9% w/w cream formulation, is < 1% of the radioactive dose, following either single or multiple doses under conditions of clinical use, that included shaving within 2 hr before radiolabeled dose application in addition to other forms of cutting or plucking and tweezing to remove facial hair. Following twice daily application of 0.5 g of the cream (total dose 1.0 g/day; 139 mg as anhydrous eflornithine hydrochloride), under conditions of clinical use in women with unwanted facial hair (n=10), the steady-state Cmax, Ctrough and AUC12hr were approximately 10 ng/mL, 5 ng/mL, and 92 nghr/mL, respectively, expressed in terms of the anhydrous free base of eflornithine hydrochloride. At steady state, the dose-normalized peak concentrations (Cmax) and the extent of daily systemic exposure (AUC) of eflornithine following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day) is estimated to be approximately 100- and 60-fold lower, respectively, when compared to 370 mg/day once-daily oral doses. Eflornithine is not metabolized and is excreted unchanged in urine. For more Absorption, Distribution and Excretion (Complete) data for Eflornithine (8 total), please visit the HSDB record page. Metabolism / Metabolites This compound is not known to be metabolized and is primarily excreted unchanged in the urine. Biological Half-Life The terminal plasma elimination half-life of eflornithine was 3.5 hours, and the apparent steady-state plasma half-life of eflornithine was approximately 8 hours. The apparent steady-state plasma t1/2 of eflornithine was approximately 8 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Maternal intravenous eflornithine 400 mg/kg daily for 7 days did not cause any adverse serious effects in breastfed infants. After topical application, eflornithine is poorly absorbed so it is not likely to reach the bloodstream of the infant or cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants A cohort of 33 infants who were breastfed (extent not stated) by hospitalized mothers taking nifurtimox was followed in the Democratic Republic of the Congo. Thirty mothers took a full course of 30 doses of oral nifurtimox 15 mg/kg daily and all received 14 doses of intravenous eflornithine 400 mg/kg daily for 7 days for human African trypanosomiasis. (sleeping sickness). Nursing mothers also took a median of 4 other concomitant medications, including amoxicillin, ciprofloxacin, metronidazole, trimethoprim-sulfamethoxazole, aspirin, and diclofenac (1 patient each); hydrocortisone, promethazine and quinine (2 patients each); levamisole (6 patients); sulfadoxine-pyrimethamine (8 patients); dipyrone (13 patients); acetaminophen (16 patients); and mebendazole (17 patients). No serious adverse events were reported in any of the breastfed infants. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Eflornithine does not specifically bind to human plasma proteins. |
| 参考文献 |
[1]. Qu N, et al. Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine. Biochem J. 2003 Oct 15;375(Pt 2):465-70.
[2]. Jansson-Löfmark R, et al. Enantiospecific reassessment of the pharmacokinetics and pharmacodynamics of oral eflornithine against late-stage Trypanosoma brucei gambiense sleeping sickness. Antimicrob Agents Chemother. 2015 Feb;59(2):1299-307. |
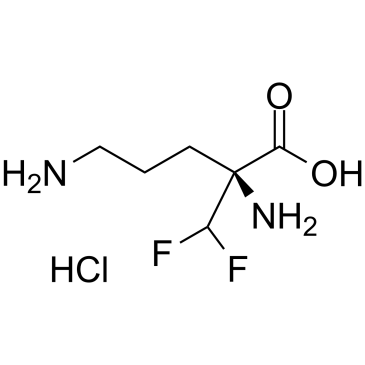
| 分子式 |
C6H15CLF2N2O3
|
|---|---|
| 分子量 |
236.644707918167
|
| 精确质量 |
218.063
|
| CAS号 |
69955-42-6
|
| 相关CAS号 |
Eflornithine;70052-12-9;Eflornithine hydrochloride hydrate;96020-91-6;Eflornithine hydrochloride;68278-23-9;L-Eflornithine;66640-93-5
|
| PubChem CID |
16048568
|
| 外观&性状 |
Light yellow to yellow solid
|
| tPSA |
89.3Ų
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
166
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C(C[C@@](C(F)F)(C(=O)O)N)CN.Cl
|
| InChi Key |
VKDGNNYJFSHYKD-FYZOBXCZSA-N
|
| InChi Code |
InChI=1S/C6H12F2N2O2.ClH/c7-4(8)6(10,5(11)12)2-1-3-9;/h4H,1-3,9-10H2,(H,11,12);1H/t6-;/m1./s1
|
| 化学名 |
(2S)-2,5-diamino-2-(difluoromethyl)pentanoic acid;hydrochloride
|
| 别名 |
L-DFMO monohydrochloride ; L-RMI71782 monohydrochloride; L-Eflornithine (monohydrochloride); 69955-42-6; L-Eflornithine monohydrochloride; (2S)-2,5-diamino-2-(difluoromethyl)pentanoic acid;hydrochloride; (S)-2,5-diamino-2-(difluoromethyl)pentanoic acid hydrochloride; L-DFMO (monohydrochloride); SCHEMBL1322403; L-α-difluoromethylornithine monohydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~200 mg/mL (~914.79 mM)
H2O : ~50 mg/mL (~228.70 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 5 mg/mL (22.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 50.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 5 mg/mL (22.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 50.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 5 mg/mL (22.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 100 mg/mL (457.39 mM) (饱和度未知) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.2258 mL | 21.1291 mL | 42.2583 mL | |
| 5 mM | 0.8452 mL | 4.2258 mL | 8.4517 mL | |
| 10 mM | 0.4226 mL | 2.1129 mL | 4.2258 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。