| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Trypanosoma; Ornithine decarboxylase
Ornithine decarboxylase (ODC) (IC50 for Trypanosoma brucei ODC: ~0.1 μM; IC50 for human ODC: ~10 μM) [1] Ornithine decarboxylase (ODC) [2][3][4] |
|---|---|
| 体外研究 (In Vitro) |
依氟鸟氨酸是鸟氨酸脱羧酶的一种特异性、不可逆的抑制剂,被认为通过抑制毛囊中的这种酶来减缓头发生长[2]。
采用Franz扩散池对依氟鸟氨酸进行体外渗透研究。当将依氟鸟氨酸乳膏涂抹在用微针预处理过的小鼠皮肤区域时,依氟鸟氨酸的毛发生长抑制活性显著增强,很可能是因为微针产生的微孔允许依氟鸟氨酸渗透到皮肤中,这在体外渗透研究中得到了证实。免疫组织化学数据显示,将依氟鸟氨酸乳膏涂抹在微针预处理的皮肤区域,皮肤和毛囊的细胞增殖也明显受到抑制。[3] 1. 抗锥虫活性:Eflornithine HCl在体外强效抑制布氏锥虫(非洲人类锥虫病的病原体)生长,最低抑菌浓度(MIC)约为0.5 μM。药物通过不可逆抑制多胺生物合成中的关键酶ODC发挥作用,导致腐胺和亚精胺耗竭,而这两种物质对锥虫的增殖和存活至关重要[1] 2. 抑制人皮肤细胞增殖:在培养的人真皮成纤维细胞和角质形成细胞中,Eflornithine HCl剂量依赖性抑制细胞增殖,IC50约为5 mM。该效应通过抑制ODC介导,导致多胺水平降低和细胞周期进程受阻[2] 3. 调节血管平滑肌细胞反应性:在分离的大鼠主动脉环中,Eflornithine HCl(10 mM)减弱苯肾上腺素诱导的血管收缩,增强乙酰胆碱诱导的血管舒张,表明其通过改变平滑肌细胞中的多胺代谢来调节血管张力[4] 4. 渗透促进剂增强局部疗效:当与渗透促进剂(如油酸)联合使用时,Eflornithine HCl(13.9% w/w)在体外人皮肤等效物中的渗透率显著提高,与单纯乳膏制剂相比,累积药物递送量增加2.3倍[3] |
| 体内研究 (In Vivo) |
过去 50 年来唯一被批准用于治疗人类非洲锥虫病的新药是依氟鸟氨酸。这种药物主要用作对 melarsoprol 耐药的布氏冈比亚锥虫病例的备用药物[1]。如果受试者面部毛发过多,则 15% 依氟鸟氨酸霜比安慰剂更能减少毛发生长。经过 24 周的治疗疗程后,58% 的依氟鸟氨酸受试者和 34% 的安慰剂受试者的面部多毛症至少有所改善[2]。当依氟鸟氨酸霜涂抹在经过微针预处理的小鼠皮肤区域时,其毛发生长抑制活性大大增强[3]。高血压14天后,依氟鸟氨酸治疗缩窄性高血压大鼠,导致收缩强度对KCI和去甲肾上腺素的反应恢复正常,并对乙酰胆碱的舒张反应恢复正常[4]。
1. 非洲人类锥虫病(HAT)模型中的疗效:在布氏锥虫感染的小鼠中,腹腔注射Eflornithine HCl(100 mg/kg,每日两次,连续7天)可实现100%存活,并完全清除血液和中枢神经系统(CNS)中的寄生虫。在III期临床试验中,静脉注射Eflornithine HCl(400 mg/kg/天,分四次给药,连续14天)对晚期HAT(CNS受累)患者的治愈率达94%[1] 2. 动物模型中的毛发生长抑制:向C57BL/6小鼠背部脱毛区域局部涂抹Eflornithine HCl乳膏(13.9% w/w),每日一次,连续21天,可显著抑制脱毛后的毛发生长,与溶媒对照组相比,毛发长度减少60%。停药后该效应具有可逆性[2] 3. 减轻缩窄诱导的高血压:在主动脉缩窄诱导高血压的大鼠中,口服Eflornithine HCl(500 mg/kg/天,连续4周)可阻止收缩压升高(治疗组145 ± 10 mmHg vs. 对照组182 ± 15 mmHg),并改善主动脉顺应性。药物还降低了血管ODC活性和多胺水平,减轻血管重塑[4] 4. 动物皮肤模型中增强局部疗效:向无毛小鼠局部涂抹含Eflornithine HCl(13.9% w/w)和油酸(5% w/w)的乳膏,与单纯乳膏相比,皮肤中药物浓度提高3.1倍,毛囊增殖抑制率达75%,显著高于单纯制剂的45%[3] |
| 酶活实验 |
In vitro permeation of eflornithine hydrochloride through mouse skin/盐酸依氟鸟氨酸体外经小鼠皮肤渗透研究[3]
如前所述,使用Franz扩散池仪完成体外渗透测定(Kumar等人,2012;Kumar et al. 2011;Naguib, Kumar, & Cui 2014)使用C57BL/6小鼠的下背皮肤。收集皮肤前24小时用电动剪发器修剪毛发。采集皮肤,用铝箔包裹,在- 20°C下保存最多一个月,并在需要时使用。在- 20°C下将皮肤冷冻(不使用冷冻保护剂)在文献中是常用的方法,并且这种皮肤样本经常用于渗透性研究(Stahl, Wohlert, & Kietzmann 2012)。Dennerlein等人表明,将新鲜切除的人体皮肤在- 20°C下冷冻和储存长达30天不会影响皮肤的渗透性(Dennerlein等人,2013年)。其他研究人员表明,将人体皮肤包裹在铝箔中并保存在- 26°C时,皮肤可保持其屏障性能长达6个月(Badran, Kuntsche, & Fahr 2009)。去除脂肪层后,将皮肤背朝上置于Franz扩散细胞上。接收器室装有5ml水,用Haake SC 100水循环器(ThermoScientific, Wellington, NH)保持在37°C。如前所述,在将毛发修饰的皮肤安装到Franz扩散池上之前,使用Dermaroller®微针辊进行处理(Kumar等人,2011;Naguib, Kumar, & Cui 2014)。将皮肤样品置于天平的平面上,微针滚轮在皮肤表面沿四个垂直方向滚动,每个方向滚动5次,共滚动20次,施加的压力为350 - 400g,在滚动滚轮的同时使用天平不断测量。皮肤扩散面积为0.64 cm2。供体室以500 μl的水注入盐酸依氟鸟氨酸4 mg,并用副膜覆盖以防止蒸发。在0、1、3、6、8和24 h后,从接收室中取出样品(150 μl),并立即补充新鲜培养基。使用高效液相色谱法对样品进行分析,方法采用先前描述的方法,并进行了修改(Saravanan et al. 2009)。色谱分析采用Agilent 1260 Infinity高效液相色谱仪,配备ZORBAX Eclipse Plus C18 (5 μm, 4.6 × 150 mm)色谱柱,流动相为乙腈-缓冲液(70%:30%,v/v)。将0.68 g磷酸一碱钾溶于1l水中制备缓冲液。流速0.8 ml/min。探测器波长为210 nm。 1. 锥虫ODC活性抑制实验:纯化重组布氏锥虫ODC,在含磷酸吡哆醛(ODC的辅因子)的实验缓冲液中稀释。将系列浓度的Eflornithine HCl(0.01–10 μM)与酶在37°C下预孵育30分钟,加入L-鸟氨酸(底物)启动反应,37°C孵育60分钟。采用气相色谱法检测反应产生的CO₂(ODC催化脱羧反应的产物),通过绘制酶活性百分比(相对于溶媒对照组)与Eflornithine HCl浓度对数的关系曲线,计算IC50值[1] 2. 人源ODC活性实验:制备重组人源ODC,实验方案与锥虫ODC实验相似,调整反应缓冲液pH值(人源ODC为7.5,锥虫ODC为8.0)和底物浓度,定量Eflornithine HCl对人源ODC的抑制效果,以确定物种特异性效力[1] |
| 细胞实验 |
皮肤组织用福尔马林(10%)缓冲溶液固定24 h,用0.1 M磷酸钠缓冲液(pH 7.4)洗涤,用分级乙醇脱水,石蜡包埋,垂直切片。切片使用苏木精-伊红(H&E)或抗5-溴-2 ' -脱氧尿苷(BrdU)抗体在德克萨斯大学奥斯汀分校戴尔儿科研究所的组织学和组织处理设施进行染色。在安乐死前30分钟,以100 μg/g体重的剂量,在磷酸盐缓冲盐水(PBS, pH 7.4, 10 mM)中腹腔注射BrdU。所有皮肤切片均在Olympus BX53显微镜下检查[3]。
1. 锥虫增殖抑制实验:布氏锥虫 bloodstream形式在改良HMI-9培养基中培养,加入梯度浓度(0.1–10 μM)的Eflornithine HCl,在37°C、5% CO₂条件下孵育。每日使用血细胞计数板测量锥虫密度,持续5天,最低抑菌浓度(MIC)定义为完全抑制锥虫生长的最低浓度[1] 2. 人皮肤细胞增殖实验:将人真皮成纤维细胞和角质形成细胞以5×10³个细胞/孔的密度接种到96孔板中,过夜培养。加入1–50 mM浓度范围的Eflornithine HCl,孵育72小时后,采用比色法检测细胞活力,计算增殖抑制的IC50值[2] 3. 血管平滑肌细胞反应性实验:将分离的大鼠主动脉环安装在含克雷布斯-林格碳酸氢盐缓冲液的器官浴中(37°C,95% O₂/5% CO₂),平衡后用Eflornithine HCl(10 mM)预处理60分钟。通过累积加入苯肾上腺素(10⁻⁸至10⁻⁴ M)诱导血管收缩,在苯肾上腺素预收缩后加入乙酰胆碱(10⁻⁸至10⁻⁴ M)评估血管舒张,采用力传感器记录血管张力变化[4] 4. 皮肤渗透实验:将人皮肤等效物安装在Franz扩散池中,角质层朝向供体室。向供体室涂抹Eflornithine HCl乳膏(含或不含渗透促进剂),受体室加入磷酸盐缓冲液(37°C,600 rpm搅拌)。在预定时间点(1–24小时)从受体室取样,通过高效液相色谱(HPLC)定量药物浓度,计算累积渗透率[3] |
| 动物实验 |
In vivo efficacy study was performed in a mouse model by monitoring the re-growth of hair in the lower dorsal skin of mice after the eflornithine cream was applied onto an area pretreated with microneedles. The skin and the hair follicles in the treated area were also examined histologically[3].
Female C57BL/6 mice (8–10 weeks old) were are ideal for examining the physiological actions during different hair cycle phases due to the occurrence of naturally synchronized hair cycles with cyclic pigmentation (Slominski, Paus, & Costantino 1991). Each experimental group was composed of 3–4 mice. Hair in the lower dorsal skin of anesthetized mice was either trimmed using an electric clipper, plucked using GiGi® Honee warm wax as previously described (Xiao et al. 2012), or chemically removed using Nair® lotion. The skin area where the hair was removed was then treated with the eflornithine hydrochloride 13.9% cream (~50 mg per mouse per treatment) using a spatula two times a day in an interval of at least 8 h for a maximum period of 36 days. A group of mice whose hair in the application site was trimmed using a clipper were also treated with the microneedle roller every time before the application of eflornithine cream as previously described (Kumar et al. 2012). Briefly, mice were placed onto the flat surface of a balance, and the microneedle roller was rolled over the marked skin surface, 10 times parallel to mouse length, with an applying pressure of 350–400 g as indicated on the balance. In control groups, the hair in mouse dorsal skin was removed by trimming, plucking, or chemical depilation with Nair®, but the area was not treated with the eflornithine cream. The hair re-growth was evaluated by taking digital photographs of the mouse skin areas for a maximum period of 36 days after the first application of the eflornithine cream. On the last day of the study, animals were euthanized, and skin samples were collected from the treated areas for immunohistochemical studies.[3] 1. Trypanosomiasis mouse model: Female BALB/c mice (6–8 weeks old) were intraperitoneally infected with 1×10⁴ Trypanosoma brucei bloodstream forms. Three days post-infection, mice were randomly divided into treatment and control groups (n=10 per group). Eflornithine HCl was dissolved in sterile saline and administered intraperitoneally at 100 mg/kg twice daily for 7 days. Control mice received sterile saline. Survival was monitored daily for 30 days, and parasite load in blood and CNS was measured by microscopic examination of blood smears and cerebrospinal fluid (CSF) samples [1] 2. Hair growth inhibition mouse model: Female C57BL/6 mice (8 weeks old) were depilated on the dorsal skin using electric clippers. Eflornithine HCl cream (13.9% w/w) or vehicle cream was applied topically to the depilated area (0.1 g/cm²) once daily for 21 days. Hair length was measured every 7 days, and skin biopsies were collected at the end of treatment to assess hair follicle proliferation by histology [2] 3. Coarctation hypertension rat model: Male Sprague-Dawley rats (200–250 g) underwent aortic coarctation surgery to induce hypertension. One week post-surgery, rats with systolic blood pressure >160 mmHg were randomized to Eflornithine HCl treatment or vehicle control (n=8 per group). Eflornithine HCl was dissolved in drinking water at a concentration of 500 mg/kg/day (based on average water intake) and administered ad libitum for 4 weeks. Systolic blood pressure was measured weekly using tail-cuff plethysmography. At the end of treatment, rats were euthanized, and aortas were excised for assessment of vascular compliance and ODC activity [4] 4. Topical efficacy enhancement mouse model: Hairless mice (6–8 weeks old) were randomly divided into two groups (n=6 per group). Group 1 received topical application of plain Eflornithine HCl cream (13.9% w/w), and Group 2 received cream containing Eflornithine HCl (13.9% w/w) plus oleic acid (5% w/w). The cream was applied to the dorsal skin (0.1 g/cm²) once daily for 14 days. Skin samples were collected 24 hours after the last dose, homogenized, and Eflornithine HCl concentration was quantified by HPLC. Hair follicle proliferation was assessed by immunohistochemical staining for Ki-67 [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administrations of eflornithine, peak plasma concentrations of eflornithine (Cmax) were achieved (Tmax) 3.5 hours post-dosing. The Cmax and AUC (area under the concentration-time curve) of eflornithine were not affected by food (high fat and high calories). Administration of crushed tablets in a standard pudding admixture had no effect on eflornithine exposure (Cmax and AUC6h). The mean percutaneous absorption of eflornithine in women with unwanted facial hair, from a 13.9% w/w cream formulation, is < 1% of the radioactive dose, following either single or multiple doses under conditions of clinical use, that included shaving within 2 hours before radiolabeled dose application in addition to other forms of cutting or plucking and tweezing to remove facial hair. Steady state was reached within four days of twice-daily application. Following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day; 139 mg as anhydrous eflornithine hydrochloride), under conditions of clinical use in women with unwanted facial hair (n=10), the steady-state Cmax, Ctrough and AUC12hr were approximately 10 ng/mL, 5 ng/mL, and 92 ng hr/mL, respectively, expressed in terms of the anhydrous free base of eflornithine hydrochloride. At steady state, the dose-normalized peak concentrations (Cmax) and the extent of daily systemic exposure (AUC) of eflornithine following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day) is estimated to be approximately 100- and 60-fold lower, respectively, when compared to 370 mg/day once-daily oral doses. This compound is not known to be metabolized and is primarily excreted unchanged in the urine. Eflornithine volume of distribution (Vz/F) is 24.3 L. The clearance (CL/F) of eflornithine is 5.3 L/h. The mean percutaneous absorption of eflornithine in women with unwanted facial hair, from a 13.9% w/w cream formulation, is < 1% of the radioactive dose, following either single or multiple doses under conditions of clinical use, that included shaving within 2 hr before radiolabeled dose application in addition to other forms of cutting or plucking and tweezing to remove facial hair. Following twice daily application of 0.5 g of the cream (total dose 1.0 g/day; 139 mg as anhydrous eflornithine hydrochloride), under conditions of clinical use in women with unwanted facial hair (n=10), the steady-state Cmax, Ctrough and AUC12hr were approximately 10 ng/mL, 5 ng/mL, and 92 nghr/mL, respectively, expressed in terms of the anhydrous free base of eflornithine hydrochloride. At steady state, the dose-normalized peak concentrations (Cmax) and the extent of daily systemic exposure (AUC) of eflornithine following twice-daily application of 0.5 g of the cream (total dose 1.0 g/day) is estimated to be approximately 100- and 60-fold lower, respectively, when compared to 370 mg/day once-daily oral doses. Eflornithine is not metabolized and is excreted unchanged in urine. For more Absorption, Distribution and Excretion (Complete) data for Eflornithine (8 total), please visit the HSDB record page. Metabolism / Metabolites This compound is not known to be metabolized and is primarily excreted unchanged in the urine. Biological Half-Life The terminal plasma elimination half-life of eflornithine was 3.5 hours, and the apparent steady-state plasma half-life of eflornithine was approximately 8 hours. The apparent steady-state plasma t1/2 of eflornithine was approximately 8 hours. 1. Absorption: Intravenous administration of Eflornithine HCl (400 mg/kg/day) in HAT patients results in peak plasma concentrations (Cmax) of ~150 μg/mL within 1 hour. Topical application of 13.9% cream leads to minimal systemic absorption (<1% of applied dose) in humans, with plasma concentrations below the detection limit (<0.1 μg/mL) [1][2] 2. Distribution: The drug distributes widely into tissues, including the CNS (cerebrospinal fluid concentration ~20% of plasma concentration after intravenous administration), which is critical for treating late-stage HAT with CNS involvement [1] 3. Metabolism: Eflornithine HCl is not metabolized significantly in humans; it undergoes minimal biotransformation to inactive metabolites [1][2] 4. Excretion: The majority of the drug is excreted unchanged in urine, with a renal clearance of ~100 mL/min in humans. The plasma elimination half-life (t1/2) is ~3 hours after intravenous administration and ~8 hours after oral administration [1] 5. Oral bioavailability: Oral bioavailability of Eflornithine HCl is ~40% in humans, due to incomplete absorption in the gastrointestinal tract [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Maternal intravenous eflornithine 400 mg/kg daily for 7 days did not cause any adverse serious effects in breastfed infants. After topical application, eflornithine is poorly absorbed so it is not likely to reach the bloodstream of the infant or cause any adverse effects in breastfed infants. ◉ Effects in Breastfed Infants A cohort of 33 infants who were breastfed (extent not stated) by hospitalized mothers taking nifurtimox was followed in the Democratic Republic of the Congo. Thirty mothers took a full course of 30 doses of oral nifurtimox 15 mg/kg daily and all received 14 doses of intravenous eflornithine 400 mg/kg daily for 7 days for human African trypanosomiasis. (sleeping sickness). Nursing mothers also took a median of 4 other concomitant medications, including amoxicillin, ciprofloxacin, metronidazole, trimethoprim-sulfamethoxazole, aspirin, and diclofenac (1 patient each); hydrocortisone, promethazine and quinine (2 patients each); levamisole (6 patients); sulfadoxine-pyrimethamine (8 patients); dipyrone (13 patients); acetaminophen (16 patients); and mebendazole (17 patients). No serious adverse events were reported in any of the breastfed infants. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. 1. Systemic toxicity (intravenous/oral administration): In HAT patients treated with intravenous Eflornithine HCl, common adverse events include diarrhea (35%), vomiting (28%), anemia (22%), and thrombocytopenia (18%). These effects are reversible and dose-related, resolving after treatment discontinuation. No severe hepatotoxicity or nephrotoxicity was reported [1] 2. Topical toxicity: Topical application of Eflornithine HCl cream is well-tolerated in humans, with mild local adverse events (e.g., pruritus, erythema, stinging) reported in <10% of patients. No systemic toxicity was observed with long-term topical use (up to 6 months) [2][3] 3. Plasma protein binding: Eflornithine HCl has low plasma protein binding (~10%) in humans, as determined by equilibrium dialysis [1] 4. Safety in animal models: In rats treated with oral Eflornithine HCl (500 mg/kg/day for 4 weeks), no significant changes in liver function tests (ALT, AST) or renal function (creatinine, BUN) were observed. Histopathological examination of major organs (liver, kidney, heart) showed no toxic lesions [4] |
| 参考文献 | |
| 其他信息 |
An inhibitor of ornithine decarboxylase, the rate limiting enzyme of the polyamine biosynthetic pathway.
See also: Eflornithine (annotation moved to); Eflornithine Hydrochloride (annotation moved to). \nEflornithine is a fluoroamino acid that is ornithine substituted by a difluoromethyl group at position 2. It has a role as a trypanocidal drug. It is a fluoroamino acid and an alpha-amino acid. It is functionally related to an ornithine. Eflornithine is an irreversible ornithine decarboxylase inhibitor originally developed as a treatment for human African trypanosomiasis. Further research has also implicated ornithine decarboxylase in other conditions like facial hirsutism and cancer, especially when ornithine decarboxylase is highly upregulated in tumor cells. Additionally, ornithine decarboxylase is activated by c-myc or interacts with ras, both very well-known oncogenes, thus increasing the interest in targeting ornithine carboxylase as a potential cancer treatment. In 1960 and 2000, the FDA approved eflornithine under the brand names ORNIDYL and VANIQUA for the treatment of African trypanosomiasis and hirsutism, respectively, but has since been discontinued. Subsequently, on December 14, 2023, the FDA approved eflornithine again but under the brand name IWILFIN as an oral maintenance therapy to reduce the risk of relapse in adult and pediatric patients with high-risk neuroblastoma who have demonstrated at least a partial response to prior multiagent, multimodality therapy, including anti-GD2 immunotherapy. This approval is based on positive results obtained from a multi-site, single-arm, externally controlled study of children with high-risk neuroblastoma, where a 52% reduction in the risk of relapse and a 68% reduction in the risk of death were observed. Eflornithine is an Antiprotozoal and Decarboxylase Inhibitor. The mechanism of action of eflornithine is as a Decarboxylase Inhibitor. Eflornithine is a difluoromethylated ornithine compound with antineoplastic activity. Eflornithine irreversibly inhibits ornithine decarboxylase, an enzyme required for polyamine biosynthesis, thereby inhibiting the formation and proliferation of tumor cells. Polyamines are involved in nucleosome oligomerization and DNA conformation, creating a chromatin environment that stimulates neoplastic transformation of cells. This agent has been shown to induce apoptosis in leiomyoma cells. (NCI04) An inhibitor of ornithine decarboxylase, the rate limiting enzyme of the polyamine biosynthetic pathway. See also: Eflornithine Hydrochloride (has salt form). Drug Indication Eflornithine is indicated to reduce the risk of relapse in adult and pediatric patients with high-risk neuroblastoma (HRNB) who have demonstrated at least a partial response to prior multiagent, multimodality therapy including anti-GD2 immunotherapy. It was also previously indicated for the treatment of female hirsutism and African trypanosomiasis but has since been discontinued. FDA Label Treatment of facial hirsutism in women. Mechanism of Action Eflornithine is an irreversible inhibitor of the enzyme ornithine decarboxylase (ODC), the first and rate-limiting enzyme in the biosynthesis of polyamines and a transcriptional target of MYCN. Polyamines are involved in the differentiation and proliferation of mammalian cells and are important for neoplastic transformation. There are no studies examining the inhibition of the enzyme ornithine decarboxylase (ODC) in human skin following the application of topical eflornithine. However, there are studies in the literature that report the inhibition of ODC activity in skin following oral eflornithine. It is postulated that topical eflornithine hydrochloride irreversibly inhibits skin ODC activity. This enzyme is necessary in the synthesis of polyamines. Animal data indicate that inhibition of ornithine decarboxylase inhibits cell division and synthetic functions, which affect the rate of hair growth. VANIQA has been shown to retard the rate of hair growth in non-clinical and clinical studies. Eflornithine (alpha-difluoromethylornithine) hydrochloride has hair growth retarding properties. The mechanism(s) by which topically applied eflornithine hydrochloride reduces hair growth has not been fully elucidated. Results of several studies using oral eflornithine hydrochloride indicate that the drug may inhibit ornithine decarboxylase (ODC), an enzyme that catalyzes the biosynthesis of intracellular polyamines required for cell division and differentiation. Limited animal data indicate that such inhibition of cell division and differentiation may affect the rate of hair growth. The manufacturer of topical eflornithine hydrochloride states that there are no published studies in humans on the ODC inhibitory potential of topical eflornithine hydrochloride. 1. Indications: Eflornithine HCl is approved for two main indications: (1) Treatment of late-stage human African trypanosomiasis (HAT, sleeping sickness) caused by Trypanosoma brucei gambiense; (2) Topical treatment of unwanted facial hair in women (marketed as Vaniqa® cream, 13.9% w/w) [1][2] 2. Mechanism of action: Eflornithine HCl is an irreversible, mechanism-based inhibitor of ornithine decarboxylase (ODC), the first and rate-limiting enzyme in the biosynthesis of polyamines (putrescine, spermidine, spermine). Polyamines are essential for cell proliferation, differentiation, and survival; inhibition of ODC leads to polyamine depletion and suppression of target cell growth (e.g., trypanosomes, hair follicle cells, vascular smooth muscle cells) [1][2][4] 3. Clinical development: For HAT, Eflornithine HCl replaced toxic arsenical drugs as the first-line treatment for late-stage disease, significantly improving patient survival. For topical use, it is the only FDA-approved drug for unwanted facial hair growth, offering a non-invasive alternative to cosmetic procedures [1][2] 4. Formulations: Available formulations include intravenous injection (200 mg/mL), oral tablets (500 mg), and topical cream (13.9% w/w). The topical cream is often combined with penetration enhancers (e.g., oleic acid) to improve skin permeation and efficacy [3] 5. Limitations: For HAT, intravenous administration requires skilled healthcare personnel and prolonged treatment (14 days), limiting access in resource-poor regions. For topical use, hair growth inhibition is partial and requires continuous treatment to maintain effects [1][2] |
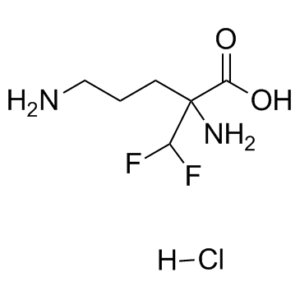
| 分子式 |
C6H12N2O2F2.HCL
|
|---|---|
| 分子量 |
218.62942
|
| 精确质量 |
236.073
|
| CAS号 |
68278-23-9
|
| 相关CAS号 |
Eflornithine;70052-12-9;Eflornithine hydrochloride hydrate;96020-91-6;L-Eflornithine monohydrochloride;69955-42-6;L-Eflornithine;66640-93-5
|
| PubChem CID |
57004
|
| 外观&性状 |
White to light yellow solid powder
|
| 沸点 |
347ºC at 760 mmHg
|
| 熔点 |
181-184°C
|
| 闪点 |
163.7ºC
|
| LogP |
1.975
|
| tPSA |
89.34
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
13
|
| 分子复杂度/Complexity |
166
|
| 定义原子立体中心数目 |
0
|
| SMILES |
NC(CCCN)(C(F)F)C(O)=O.[H]Cl
|
| InChi Key |
VKDGNNYJFSHYKD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C6H12F2N2O2.ClH/c7-4(8)6(10,5(11)12)2-1-3-9;/h4H,1-3,9-10H2,(H,11,12);1H
|
| 化学名 |
2,5-diamino-2-(difluoromethyl)pentanoic acid;hydrochloride
|
| 别名 |
DFMO hydrochloride; MDL71782 hydrochloride; RMI71782 hydrochloride; α-difluoromethylornithine hydrochloride; MDL-71782 hydrochloride; RMI-71782 hydrochloride; α-difluoromethylornithine hydrochloride; MDL 71782 hydrochloride; RMI 71782 hydrochloride; α-difluoromethylornithine hydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Typically soluble in DMSO (e.g. 10 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5739 mL | 22.8697 mL | 45.7394 mL | |
| 5 mM | 0.9148 mL | 4.5739 mL | 9.1479 mL | |
| 10 mM | 0.4574 mL | 2.2870 mL | 4.5739 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Digital photographs of C57BL/6 mouse dorsal skin with and without treatment with the Vaniqa eflornithine cream (13.9%) for up to 36 days. |
|---|
 In vitropermeation of eflornithine hydrochloride in a solution through a mouse skin area where the hair was trimmed (without microneedle), or trimmed and then treated with microneedles (with microneedle). Data shown are mean ± S.D. (n = 3).Drug Deliv.2016 Jun;23(5):1495-501. |
 Representative micrographic pictures of skin samples after anti-BrdU staining (A) or H&E staining (B). Scale bar = 2 mm.Drug Deliv.2016 Jun;23(5):1495-501. |