| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
protease: Cathepsin B; Cathepsin L; Cathepsin H; Ser/Thr Protease; Mpro
Target: Inhibits bovine trypsin (Ki = 72 nM), bovine α-chymotrypsin (weaker inhibition, no specific Ki reported), human plasmin (Ki = 35 nM), bovine thrombin (weak inhibition, no specific Ki reported), and bovine spleen cathepsin B (Ki = 3.4 μM) [1] Inhibits proteases involved in tubulin degradation in plant cells (no specific Ki/IC50 reported) [2] Inhibits SARS-CoV-2 main protease (Mpro, IC50 = 127.2 μM) [5] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:亮肽素由多种放线菌产生,强烈抑制蛋白水解。半硫酸亮抑酶肽可在分离过程中保护微管蛋白免受内源蛋白水解活性的影响,从而提高微管蛋白的纯度。 Leupeptin hemisulfate 可恢复细胞悬浮培养物中高达 50% 的乙型肝炎表面抗原 (HBsAg) 表达。细胞测定:在 MRC-C 细胞培养物中,亮肽素可阻止人类冠状病毒株 229E 的增殖。 Leupeptin 在空斑测试中的 IC50 值为 0.4 μg/mL,而宿主细胞的生长不受 50 μg/mL Leupeptin 的影响。在单周期生长实验中,只有在感染后 2 小时内添加亮肽素 (100 μg/mL) 才会降低病毒产量,表明其在病毒复制的早期阶段发挥作用。
- 以72 nM的Ki值抑制牛胰蛋白酶活性;在10 μg/ml浓度下,可完全抑制人纤溶酶诱导的纤维蛋白溶解。对牛α-胰凝乳蛋白酶和凝血酶的抑制作用较弱。在100 μg/ml浓度下,对兔肾细胞或小鼠成纤维细胞的生长无影响[1] - 在烟草BY-2悬浮细胞中,100 μM的Leupeptin可抑制参与微管蛋白降解的蛋白酶活性,通过SDS-PAGE和光密度分析显示,处理24小时后微管蛋白降解减少[2] - 在体外抑制人前列腺癌PC-3细胞的生长,经Leupeptin处理(浓度未明确)后,细胞增殖降低,血管生成因子(VEGF)和溶骨因子(MMP-9)的表达通过Western blot和RT-PCR检测显示下调[3] - 在Vero细胞中,Leupeptin(0.06–200 μM)处理72小时后,可减少SARS-CoV-2病毒RNA复制,EC50为42.34 μM;同时以127.2 μM的IC50抑制Mpro活性[5] |
| 体内研究 (In Vivo) |
动物对亮肽素具有良好的耐受性,并且以剂量依赖性方式使总组织提取物和溶酶体富集部分(LE 部分)中的 LC3b-II 显着增加。在电子显微镜 (EM) 水平上,亮肽素诱导肝细胞中电子致密囊泡结构的积累,在治疗 (40 mg/kg) 后 60 分钟即可观察到。结果表明,亮肽素通过保护LC3b-II蛋白免于在溶酶体内被降解来增强体内LC3b-II水平,因此基于亮肽素的测定可潜在用于研究小鼠巨自噬的动态。
巨噬是一个高度保守的分解代谢过程,对哺乳动物的器官稳态至关重要。然而,直接测量体内巨噬活性(或通量)的方法有限。在本研究中,我们开发了一种定量的巨噬通量测定方法,该方法是在给予蛋白酶抑制剂leupeptin后,在体内测量LC3b蛋白的周转。利用这种方法,我们表征了小鼠不同器官的基础巨噬通量。我们发现,经leupeptin治疗后LC3b的积累率在肝脏最大,在脾脏最低。有趣的是,我们发现ATG8/LC3b的同系物LC3a和LC3b相互作用蛋白p62的降解动力学与LC3b相似。然而,LC3b相关蛋白GABARAP和GATE-16在小鼠肝脏中并没有快速翻转,这意味着不同的LC3b同源物可能通过不同的机制促进巨噬。根据我们的实验,营养饥饿增加了巨噬通量,而在饥饿一段时间后重新喂食动物显著抑制了巨噬通量。我们还证实,与野生型小鼠相比,beclin 1杂合小鼠的基础巨噬通量降低。这些结果说明了我们基于leupeptin的检测在研究小鼠巨噬动力学方面的有效性。[4] - 在人前列腺癌骨转移小鼠模型(胫骨注射PC-3细胞)中,Leupeptin(10 mg/kg,腹腔注射,每日一次,持续4周)可减少肿瘤生长,减轻溶骨性病变,并抑制血管生成(CD31阳性血管减少)[3] - 在C57BL/6小鼠中,单次腹腔注射Leupeptin(9、18或36 mg/kg)后,肝、肾和心脏组织中LC3B-II/LC3B-I比值呈剂量依赖性增加,表明自噬流受到抑制[4] |
| 酶活实验 |
Mpro酶活性抑制试验。 [5]
共20个 将去离子水中的mM亮蛋白半硫酸酯稀释至2 mM至31.25 μM,含25 mM Tris缓冲液(pH 8.0). 一种30μl的抑制剂溶液,在25 mM Tris缓冲液(pH 8.0)首先与10 μl 100 μM肽底物(Dabcyl TSAVLQ↓SGFRKMK Edans;GenScript)。接下来,10 μl,最终浓度为200 将nM Mpro加入到板中。用360的激发波长测量相对荧光单位(RFU)值 nm,发射波长490 通过使用SpectraMax Paradigm多模检测平台(Molecular Devices,USA),在37°C下在nm下进行1小时。实验一式三份。用MS Excel计算酶活性反应速率和抑制率。使用GraphPad Prism 8.0绘制抑制曲线。 体外抗病毒试验。 [5] 共20个 将去离子水中的mM亮蛋白半硫酸酯稀释至200 μM至0.06 μM含有1%FBS的DMEM。在96孔板中培养过夜的Vero细胞以0.01的感染倍数(MOI)感染病毒2小时。取出培养基,然后向细胞中加入新鲜的含药物培养基。48小时后,将细胞在裂解缓冲液中裂解。病毒核糖核酸在100 μl的细胞上清液通过逆转录聚合酶链式反应(RT-PCR)进行定量。72小时后,通过显微镜观察细胞病变效应的变化。实验一式三份。使用MS Excel和GraphPad Prism 8.0对实验结果进行处理。 - 胰蛋白酶抑制实验:将牛胰蛋白酶与底物(苯甲酰-L-精氨酸-对硝基苯胺)在缓冲液中于37°C孵育,通过分光光度法测定对硝基苯胺的释放速率。向反应体系中加入Leupeptin,根据剂量-反应曲线计算抑制常数(Ki)[1] - 纤溶酶抑制实验:将人纤溶酶与纤维蛋白原混合,监测纤维蛋白溶解过程。加入不同浓度的Leupeptin,测定抑制50%纤维蛋白溶解所需的浓度[1] - SARS-CoV-2 Mpro实验:将重组Mpro与荧光底物(Dabcyl-KTSAVLQSGFR-Edans)及不同浓度的Leupeptin共同孵育,通过测定荧光强度变化计算IC50,反映底物切割的减少[5] |
| 细胞实验 |
Leupeptin 抑制 MRC-C 细胞培养物中人类冠状病毒株 229E 的增殖。 Leupeptin在空斑测试中的IC50值为0.4 μg/mL,在50 μg/mL时,它对宿主细胞的生长能力没有影响。在感染后两小时内添加亮肽素 (100 μg/mL) 仅在单周期生长实验中抑制病毒产量,表明它作用于病毒复制的早期阶段。
- 植物细胞蛋白酶实验:裂解烟草BY-2悬浮细胞,将细胞提取物与纯化的微管蛋白在有无100 μM Leupeptin的条件下孵育,通过SDS-PAGE和光密度分析检测微管蛋白降解情况[2] - 前列腺癌细胞实验:体外培养PC-3细胞,经Leupeptin处理(浓度未明确)后,通过MTT法测定细胞增殖,通过Western blot和RT-PCR分析VEGF/MMP-9的表达[3] - SARS-CoV-2复制实验:Vero细胞感染SARS-CoV-2后,用Leupeptin(0.06–200 μM)处理72小时,提取病毒RNA,通过RT-PCR定量病毒载量[5] |
| 动物实验 |
C57BL/6NCrl male mice
20 mg/kg i.p. Mice received i.p. injections of 0.5 ml sterile Phosphate Buffered Saline (PBS, GIBCO 10010) or 0.5 ml PBS containing 9–40 mg/kg leupeptin hemisulfate. In other experiments (Fig. 1), mice alternatively received 28–112 mg/kg chloroquine in PBS or 0.1–0.3 mg/kg Bafilomycin B1 in PBS. After injection, the mice were returned to their cages and provided free access to food and water unless they were being subjected to calorie-starvation for experimental purposes. At specified time points after injection, the mice were euthanized and their solid organs were manually dissected and flash frozen in liquid nitrogen. In experiments in which macroautophagic flux was compared between treatments (for example starvation versus fed; Fig. 7) or genotypes (beclin 1+/+ versus beclin 1−/−, Fig. 8), care was taken to dissect the different experimental groups in parallel to ensure they were exposed to leupeptin for equal amounts of time. [4] - Bone metastasis model: Nude mice were anesthetized, and PC-3 cells (1×10⁵) were injected into the left tibia. After 1 week, leupeptin (10 mg/kg) or vehicle was administered via intraperitoneal injection once daily for 4 weeks. Mice were euthanized, and tibias were harvested for histopathological analysis and quantification of tumor area [3] - Autophagy flux assay: C57BL/6 mice were injected intraperitoneally with a single dose of leupeptin (9, 18, or 36 mg/kg) dissolved in saline. After 4 hours, mice were euthanized, and liver, kidney, and heart tissues were collected for Western blot analysis of LC3B-II/LC3B-I ratio [4] |
| 参考文献 |
[1]. Biological activities of leupeptins. J Antibiot (Tokyo). 1969 Nov;22(11):558-68. [4]. Characterization of macroautophagic flux in vivo using a leupeptin-based assay. Autophagy. 2011 Jun;7(6):629-42. |
| 其他信息 |
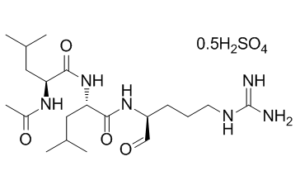
Leupeptin is a tripeptide composed of N-acetylleucyl, leucyl and argininal residues joined in sequenceby peptide linkages. It is an inhibitor of the calpains, a family of calcium-activated proteases which promote cell death. It has a role as a serine protease inhibitor, a bacterial metabolite, a cathepsin B inhibitor, a calpain inhibitor and an EC 3.4.21.4 (trypsin) inhibitor. It is a tripeptide and an aldehyde. It is a conjugate base of a leupeptin(1+).
Leupeptin has been reported in Streptomyces lavendulae, Streptomyces exfoliatus, and other organisms with data available. The requirement for proteinase inhibitors during the chromatographic isolation of tubulin from cultured cells of rose (Rosa sp. cv. Paul's scarlet) was examined by NadodecylSO4-polyacrylamide gel electrophoresis, electron microscopy and immunoblotting. Tubulin fractions isolated in the absence of proteinase inhibitors showed substoichiometric ratios of alpha-subunit to beta-subunit, and low molecular weight polypeptides, one (approximately 32 Kd) of which coassembled with polymers. Electron microscopy revealed polymorphic structures, including C- and S-shaped ribbons and free protofilaments. Immunoblotting experiments with IgGs to the individual alpha- and beta-subunits showed that some of the low molecular weight polypeptides were fragments of proteolytically degraded subunits. The use of low micromolar concentrations of the synthetic proteinase inhibitors leupeptin hemisulfate and pepstatin A protected tubulin from endogenous proteolytic activities during the isolation procedure and resulted in increased tubulin purity.[2] Soybean cell suspension cultures were transformed using Agrobacterium tumefaciens harboring pHBS/pHER constructs to express hepatitis B surface antigen (HBsAg). The transformed colonies were selected and analyzed for the expression of HBsAg by PCR, reverse transcription (RT) PCR, Western blot and ELISA analysis. The maximum expression of 700 ng/g F.W. was noted in pHER transformed cells. The highest expressing colonies were used to initiate the cell suspension cultures and the expression of HBsAg was estimated periodically. The expression levels were reduced drastically in cell suspension cultures compared to the colonies maintained on semi-solid medium. Various parameters were studied to maximize the cell growth and to retain the expression levels. The supplementation of culture medium with a protease inhibitor, leupeptin hemisulfate could restore up to 50% of HBsAg expression in cell suspension cultures. This is the first report to investigate the possible cause and solution to the loss of recombinant protein expression levels in plant cell suspension cultures.[3] Coronavirus disease 2019 (COVID-19) has caused huge deaths and economic losses worldwide in the current pandemic. The main protease (Mpro) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is thought to be an ideal drug target for treating COVID-19. Leupeptin, a broad-spectrum covalent inhibitor of serine, cysteine, and threonine proteases, showed inhibitory activity against Mpro, with a 50% inhibitory concentration (IC50) value of 127.2 μM in vitro in our study here. In addition, leupeptin can also inhibit SARS-CoV-2 in Vero cells, with 50% effective concentration (EC50) values of 42.34 μM. More importantly, various strains of streptomyces that have a broad symbiotic relationship with medicinal plants can produce leupeptin and leupeptin analogs to regulate autogenous proteases. Fingerprinting and structure elucidation using high-performance liquid chromatography (HPLC) and high-resolution mass spectrometry (HRMS), respectively, further proved that the Qing-Fei-Pai-Du (QFPD) decoction, a traditional Chinese medicine (TCM) formula for the effective treatment of COVID-19 during the period of the Wuhan outbreak, contains leupeptin. All these results indicate that leupeptin at least contributes to the antiviral activity of the QFPD decoction against SARS-CoV-2. This also reminds us to pay attention to the microbiomes in TCM herbs as streptomyces in the soil might produce leupeptin that will later infiltrate the medicinal plant. We propose that plants, microbiome, and microbial metabolites form an ecosystem for the effective components of TCM herbs.[5] - Leupeptin is a tripeptide protease inhibitor isolated from Streptomyces roseus. It is soluble in water and stable in acidic conditions [1] - It acts as a reversible competitive inhibitor of serine and cysteine proteases by binding to the active site of target enzymes [1] |
| 分子式 |
C20H38N6O4.1/2H2SO4
|
|---|---|
| 分子量 |
475.59
|
| 精确质量 |
950.56
|
| 元素分析 |
C, 50.51; H, 8.27; N, 17.67; O, 20.18; S, 3.37
|
| CAS号 |
103476-89-7
|
| 相关CAS号 |
Leupeptin;55123-66-5;Leupeptin Ac-LL;24365-47-7; Leupeptin hemisulfate;103476-89-7; 39740-82-4 (HCl); 55123-66-5; 1082207-96-2 (hemisulfate hydrate); 103476-89-7 (hemisulfate)
|
| PubChem CID |
72429
|
| 序列 |
N-acetyl-L-leucyl-L-leucyl-L-argininal compound with N-acetyl-L-leucyl-L-leucyl-L-argininal sulfuric acid
|
| 短序列 |
Ac-LLR-CHO; Ac-Leu-Leu-Arg-al.Ac-Leu-Leu-Arg-al.H2SO4
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 折射率 |
1.557
|
| 来源 |
Microbial Metabolite
|
| LogP |
1.16
|
| tPSA |
166.27
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
602
|
| 定义原子立体中心数目 |
3
|
| SMILES |
S(=O)(=O)(O[H])O[H].O=C([C@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C(C([H])([H])[H])=O)N([H])[C@]([H])(C(N([H])[C@]([H])(C([H])=O)C([H])([H])C([H])([H])C([H])([H])/N=C(\N([H])[H])/N([H])[H])=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H].O=C([C@]([H])(C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H])N([H])C(C([H])([H])[H])=O)N([H])[C@]([H])(C(N([H])[C@]([H])(C([H])=O)C([H])([H])C([H])([H])C([H])([H])/N=C(\N([H])[H])/N([H])[H])=O)C([H])([H])C([H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
CIPMKIHUGVGQTG-VFFZMTJFSA-N
|
| InChi Code |
InChI=1S/2C20H38N6O4.H2O4S/c2*1-12(2)9-16(24-14(5)28)19(30)26-17(10-13(3)4)18(29)25-15(11-27)7-6-8-23-20(21)22;1-5(2,3)4/h2*11-13,15-17H,6-10H2,1-5H3,(H,24,28)(H,25,29)(H,26,30)(H4,21,22,23);(H2,1,2,3,4)/t2*15-,16-,17-;/m00./s1
|
| 化学名 |
(2S)-2-acetamido-N-[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]-4-methylpentanamide;sulfuric acid
|
| 别名 |
NK-381; Leupeptin hemisulfate; 103476-89-7; Leupeptin; Leupeptin hemisulfate anhydrous; Leupeptin hemisulfate salt; UNII-05V9Y5208M; 05V9Y5208M; L-Leucinamide, N-acetyl-L-leucyl-N-((1S)-4-((aminoiminomethyl)amino)-1-formylbutyl)-, sulfate (2:1); NK 381; Leupeptin hemisulfate; NK381;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (210.27 mM) in PBS, 澄清溶液; 超声助溶。 (<60°C).
配方 2 中的溶解度: ~83 mg/mL (175 mM) in H2O 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1027 mL | 10.5133 mL | 21.0265 mL | |
| 5 mM | 0.4205 mL | 2.1027 mL | 4.2053 mL | |
| 10 mM | 0.2103 mL | 1.0513 mL | 2.1027 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。