| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
- PI3K/Akt/mTOR signaling pathway: Inhibits phosphorylation of PI3K, Akt, and mTOR in AGS gastric cancer cells [2]
- MAPK signaling pathway (ERK, JNK, p38): Activates phosphorylation of ERK, JNK, and p38 in AGS cells [2] - Nuclear factor erythroid 2-related factor 2 (Nrf2): Activates Nrf2 nuclear translocation and upregulates its downstream target genes (HO-1, NQO1) in PC12 cells [3] - Zinc finger E-box-binding homeobox 1 (Zeb1): Downregulates Zeb1 protein expression in osteosarcoma cells (MG-63, U2OS) [5] - Oxidative stress/inflammatory mediators (ROS, TNF-α, IL-6, NF-κB): Inhibits ROS production and NF-κB activation, reduces TNF-α/IL-6 secretion [1,3] . |
|---|---|
| 体外研究 (In Vitro) |
柚皮苷抑制 NF-κ B 信号通路的激活。在 HBZY-1 细胞中,柚皮素可防止高糖引起的氧化应激损伤、炎症反应和增殖[1]。柚皮苷以时间和剂量依赖性方式抑制 AGS 癌细胞生长。在柚皮苷处理的 AGS 细胞中,PI3K 及其激活的下游靶标 p-Akt 和 p-mTOR 的磷酸化在 2 mM 时显着降低。在 AGS 细胞中,柚皮苷会导致自噬性细胞死亡。在 AGS 细胞中,柚皮苷触发自噬相关蛋白[2]。柚皮苷可保护 PC12 细胞免受 3-NP 神经毒性的影响。当 3-NP 诱导的 PC12 细胞用柚皮苷处理时,乳酸脱氢酶的释放减少。通过提高还原型谷胱甘肽的量和酶抗氧化剂的活性,柚皮苷疗法可以改善抗氧化防御[3]。
1. 对高糖诱导足细胞损伤的保护作用(糖尿病肾病模型): - 小鼠足细胞在高糖培养基(30 mM葡萄糖)中培养,并用Naringin(25、50、100 μM)处理48小时。与高糖组相比: - 细胞内ROS水平(DCFH-DA探针)分别降低28.3%±3.1%(25 μM)、45.6%±2.8%(50 μM)、62.1%±3.5%(100 μM)[1] - 炎症因子分泌(ELISA)降低:TNF-α降低24.5%(50 μM)、41.2%(100 μM),IL-6降低21.8%(50 μM)、38.7%(100 μM)[1] - 足细胞标志蛋白(nephrin)表达(Western blot)升高1.4倍(50 μM)、1.8倍(100 μM)[1] 2. 对AGS胃癌细胞的增殖抑制及自噬诱导作用: - Naringin以剂量依赖性抑制AGS细胞增殖(MTT法):48小时IC50=85.7 μM,72小时IC50=62.3 μM[2] - Naringin(50、100 μM)处理48小时: - 诱导自噬:LC3-II/LC3-I比值升高1.9倍(50 μM)、2.7倍(100 μM),p62蛋白降低42%(50 μM)、65%(100 μM)(Western blot)[2] - 抑制PI3K/Akt/mTOR通路:p-PI3K、p-Akt、p-mTOR降低35%-60%(100 μM)[2] - 激活MAPK通路:p-ERK、p-JNK、p-p38升高1.5-2.2倍(100 μM)[2] 3. 对3-NP诱导PC12细胞损伤的神经保护作用: - PC12细胞用3-硝基丙酸(3-NP,5 mM)+Naringin(25、50、100 μM)处理24小时: - 线粒体功能改善:ATP含量升高1.3倍(50 μM)、1.7倍(100 μM),线粒体膜电位(ΔΨm,JC-1染色)升高40%(50 μM)、65%(100 μM)[3] - Nrf2信号激活:Nrf2核转位升高2.1倍(100 μM),下游抗氧化酶(SOD、CAT、GSH-Px)活性升高1.4-1.8倍(100 μM)[3] - ROS水平(DCFH-DA)降低38%(50 μM)、55%(100 μM)[3] 4. 对骨肉瘤细胞(MG-63、U2OS)的抗转移作用: - Naringin(20、40、80 μM)抑制MG-63细胞迁移(Transwell法):40 μM抑制32%,80 μM抑制58%;侵袭抑制:40 μM抑制28%,80 μM抑制52%[5] - 下调Zeb1(促进EMT的转录因子):Zeb1蛋白表达降低45%(40 μM)、70%(80 μM)(Western blot),导致EMT标志蛋白vimentin降低、E-钙黏蛋白升高[5] 。 |
| 体内研究 (In Vivo) |
柚皮苷治疗可显着减少糖尿病大鼠的肾损伤并导致体重大幅增加。在糖尿病大鼠中,柚皮苷成功减少胶原沉积和肾间质纤维化。柚皮苷治疗可能会导致 ROS 和 MDA 水平下降,而 SOD 和 GSH-Px 活性上升[1]。口服柚皮苷可显着增强记忆和学习能力。柚皮苷显着增强胰岛素信号通路[3]。
1. 对STZ诱导糖尿病肾病(DKD)大鼠的保护作用: - 雄性SD大鼠通过链脲佐菌素(STZ,60 mg/kg,腹腔注射)建立DKD模型。Naringin以50、100 mg/kg/天灌胃给药,连续8周: - 肾功能改善:血清肌酐从模型组的158 μmol/L降至50 mg/kg组112 μmol/L、100 mg/kg组85 μmol/L;尿白蛋白/肌酐比值(UACR)从模型组385 mg/g降至50 mg/kg组242 mg/g、100 mg/kg组156 mg/g[1] - 氧化应激降低:肾组织MDA含量降低35%(50 mg/kg)、52%(100 mg/kg);SOD活性升高1.4倍(50 mg/kg)、1.7倍(100 mg/kg)[1] - 炎症抑制:肾组织TNF-α、IL-6 mRNA降低40%-65%(100 mg/kg)(RT-PCR)[1] 2. 对高脂饮食(HFD)诱导肥胖小鼠的神经保护作用: - C57BL/6小鼠喂食HFD(60%脂肪)16周,诱导肥胖及认知障碍。Naringin(100 mg/kg/天,灌胃)在最后8周给药: - 认知功能改善:Morris水迷宫测试显示逃避潜伏期从HFD组58秒降至32秒;目标象限停留时间从22%升至45%[4] - 脑线粒体功能增强:海马ATP含量升高1.5倍;线粒体复合物I/IV活性升高1.3-1.4倍[4] - 神经元胰岛素信号激活:海马p-IRS-1(Tyr632)升高1.6倍;p-Akt升高1.8倍(Western blot)[4] - 体重及糖代谢:体重增长降低20%;空腹血糖从HFD组8.7 mmol/L降至6.2 mmol/L[4] 。 |
| 酶活实验 |
1. 抗氧化酶(SOD、CAT、GSH-Px)活性检测:
- 组织匀浆(肾、海马)或细胞裂解液用冰浴0.1 M磷酸缓冲液(pH 7.4)制备。SOD检测:0.1 mL匀浆/裂解液与2.9 mL反应缓冲液(黄嘌呤、黄嘌呤氧化酶、NBT)混合,37℃孵育40分钟,550 nm测吸光度;活性定义为抑制50% NBT还原所需酶量(U/mg蛋白)[1,3,4] - CAT检测:0.2 mL匀浆与1.8 mL磷酸缓冲液+1 mL 0.03 M H₂O₂混合,记录1分钟内240 nm吸光度下降值;活性=每分钟分解μmol H₂O₂/ mg蛋白[1,3] - GSH-Px检测:0.1 mL匀浆与GSH、H₂O₂、DTNB混合,37℃孵育5分钟,412 nm测吸光度;活性=每分钟氧化μmol GSH/ mg蛋白[3] 2. ROS检测(DCFH-DA探针): - 细胞(足细胞、PC12)接种于96孔板,用Naringin+应激源(高糖、3-NP)处理24小时。37℃孵育10 μM DCFH-DA 30分钟,PBS洗涤;酶标仪在激发波长488 nm、发射波长525 nm测荧光强度;ROS水平以相对荧光单位(RFU)表示[1,3] 3. 炎症因子ELISA检测(TNF-α、IL-6): - 收集细胞上清(足细胞)或血清/肾匀浆(大鼠)。100 μL样本加入包被抗TNF-α/IL-6抗体的ELISA孔,37℃孵育1小时;加入生物素标记二抗孵育30分钟,再加入链霉亲和素-HRP;加入TMB底物孵育15分钟,H₂SO₄终止反应;450 nm测吸光度,标准曲线计算细胞因子浓度[1] 。 |
| 细胞实验 |
1. AGS胃癌细胞增殖与自噬实验:
- 增殖(MTT):AGS细胞以5×10³个/孔接种于96孔板,用Naringin(0-200 μM)处理48/72小时;加入20 μL MTT(5 mg/mL),37℃孵育4小时;DMSO溶解甲臜,570 nm测吸光度;细胞活力=(药物组OD/对照组OD)×100%[2] - 自噬(Western blot):细胞以2×10⁶个/孔接种于6孔板,用Naringin(50、100 μM)处理48小时;含蛋白酶抑制剂的RIPA裂解液裂解细胞,30 μg蛋白经SDS-PAGE分离后转移至PVDF膜;用抗LC3、p62、p-PI3K、p-Akt、p-mTOR抗体孵育;ImageJ定量条带强度[2] - 克隆形成:细胞以2×10³个/孔接种于6孔板,用Naringin(50、100 μM)处理14天;甲醇固定,结晶紫染色;计数>50个细胞的克隆[2] 2. PC12细胞线粒体功能实验: - 细胞以1×10⁵个/孔接种于24孔板,用3-NP(5 mM)+Naringin(25-100 μM)处理24小时。线粒体膜电位(ΔΨm):5 μM JC-1孵育20分钟,PBS洗涤;酶标仪测红色(聚集态JC-1,高ΔΨm)和绿色(单体JC-1,低ΔΨm)荧光;ΔΨm=红/绿荧光比值[3] - ATP含量:细胞用ATP裂解液裂解,100 μL裂解液与ATP检测试剂混合; luminometer测发光强度,标准曲线计算ATP含量[3,4] 3. 骨肉瘤细胞迁移与侵袭实验(Transwell): - 迁移:MG-63细胞(5×10⁴个)悬浮于含Naringin(20-80 μM)的无血清培养基,加入Transwell上室;下室加10% FBS培养基;37℃孵育24小时;擦去上室细胞,下室细胞固定、结晶紫染色;显微镜下计数(每孔5个视野)[5] - 侵袭:上室预涂Matrigel(1:8稀释),其余步骤同迁移实验[5] ; |
| 动物实验 |
Rats: The rats are randomly divided into six groups: control, naringin (80 mg/kg), STZ, STZ+naringin (20 mg/kg), STZ+naringin (40 mg/kg), STZ+naringin(80 mg/kg). The rats in the STZ and STZ+naringin groups are intraperitoneally injected with STZ (65 mg/kg). The control and naringin groups are intraperitoneally injected with 0.1 M citrate buffer of same volume. After injection of STZ for 3 and 5 days, blood glucose levels are measured by tail vein puncture blood sampling. Mice: Sixty 4-week-old male mice are randomized into four groups and fed for 20 weeks with either control diet or high-fat diet chow. Mice are dosed with 100 mg/kg of naringin daily. Mice body weight and food intake are weekly measured. Following behavioral assessment, animals are deeply anesthetized with isoflurane and sacrificed by decapitation after fasting for at least 5 h. Their plasma is collected for further analysis. 1. STZ-induced diabetic kidney disease rat model: - Animals: Male SD rats (200-220 g, 8 weeks old) randomly divided into 4 groups (n=6): Normal control (NC), DKD model (STZ), Naringin low dose (50 mg/kg), high dose (100 mg/kg) [1] - DKD induction: Rats fasted for 12 hours, injected intraperitoneally with STZ (60 mg/kg, dissolved in 0.1 M citrate buffer, pH 4.5). Normal control injected with citrate buffer. Fasting blood glucose (FBG) >16.7 mmol/L after 72 hours confirmed diabetes [1] - Drug administration: Naringin dissolved in 0.5% carboxymethylcellulose (CMC) to prepare 5 and 10 mg/mL suspensions. Administered by oral gavage (10 mL/kg body weight) once daily for 8 weeks. NC and STZ groups received 0.5% CMC [1] - Sample collection: Rats fasted 12 hours, anesthetized with pentobarbital. Blood collected via abdominal aorta for serum creatinine, TNF-α/IL-6. Kidneys excised: one part fixed in 4% formalin (HE/PAS staining), one part homogenized (SOD, CAT, MDA), one part stored at -80°C (Western blot/RT-PCR) [1] 2. HFD-induced obese mouse model: - Animals: Male C57BL/6 mice (18-20 g, 6 weeks old) randomly divided into 3 groups (n=8): Normal diet (ND), HFD, HFD + Naringin (100 mg/kg) [4] - Obesity induction: ND group fed standard diet (10% fat); HFD and HFD + Naringin groups fed HFD (60% fat) for 16 weeks. Naringin (dissolved in 0.5% CMC) administered by oral gavage (10 mL/kg) once daily for the last 8 weeks [4] - Cognitive testing (Morris water maze): On days 1-5 (training), mice trained to find a hidden platform. On day 6 (probe trial), platform removed; escape latency and time in target quadrant recorded [4] - Sample collection: Mice euthanized, brains excised. Hippocampus dissected for mitochondrial function (ATP, complex activity), Western blot (insulin signaling proteins). Blood collected for fasting glucose, insulin [4] . |
| 毒性/毒理 (Toxicokinetics/TK) |
1. In vitro cytotoxicity:
- In normal cells (mouse podocytes, PC12 cells), Naringin at concentrations up to 200 μM had no significant effect on cell viability (MTT assay: viability >90% vs. control) [1,3] - In AGS and osteosarcoma cells, Naringin showed selective cytotoxicity (IC50 62.3-85.7 μM) without affecting normal cell viability [2,5] 2. In vivo toxicity: - In STZ-induced DKD rats (50-100 mg/kg Naringin, 8 weeks): No significant changes in body weight, food/water intake vs. normal control. Serum ALT, AST (liver function) and BUN (renal function) were within normal ranges; no histopathological lesions in liver/kidney [1] - In HFD-induced obese mice (100 mg/kg Naringin, 8 weeks): No mortality or abnormal behavior. Liver weight/body weight ratio, serum lipid profiles (TC, TG, LDL-C) showed no toxic changes; liver histology (HE staining) had no steatosis or inflammation [4] ; |
| 参考文献 |
|
| 其他信息 |
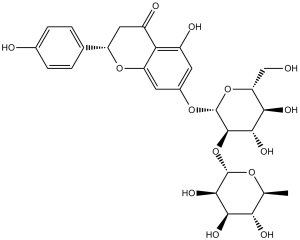
Naringin is a disaccharide derivative that is (S)-naringenin substituted by a 2-O-(alpha-L-rhamnopyranosyl)-beta-D-glucopyranosyl moiety at position 7 via a glycosidic linkage. It has a role as a metabolite, an antineoplastic agent and an anti-inflammatory agent. It is a disaccharide derivative, a dihydroxyflavanone, a member of 4'-hydroxyflavanones, a (2S)-flavan-4-one and a neohesperidoside. It is functionally related to a (S)-naringenin.
Naringin has been reported in Salvia officinalis, Citrus reticulata, and other organisms with data available. See also: Naringenin (subclass of); Drynaria fortunei root (part of). 1. Background and source: - Naringin is a natural flavanone glycoside primarily isolated from the peel of citrus fruits (e.g., grape柚, orange, lemon) and herbs (e.g., Sophora japonica). It is a major active component with antioxidant, anti-inflammatory, anticancer, and neuroprotective properties [1-5] 2. Mechanism of action: - Antioxidant: Activates Nrf2 signaling to upregulate antioxidant enzymes (SOD, CAT, GSH-Px) and scavenges ROS directly [1,3,4] - Anti-inflammatory: Inhibits NF-κB pathway to reduce pro-inflammatory cytokines (TNF-α, IL-6) [1] - Anticancer: Inhibits PI3K/Akt/mTOR pathway, activates MAPK to induce autophagy-mediated cancer cell growth inhibition; downregulates Zeb1 to suppress EMT and metastasis [2,5] - Neuroprotective: Improves brain mitochondrial function, activates neuronal insulin signaling, and reduces oxidative stress to enhance cognitive function [4] - Antidiabetic kidney protection: Reduces renal oxidative stress, inflammation, and podocyte injury to ameliorate DKD [1] 3. Therapeutic potential: - Naringin shows potential for treating chronic diseases: (1) Metabolic disorders (diabetes, obesity, DKD); (2) Cancers (gastric cancer, osteosarcoma); (3) Neurodegenerative diseases (cognitive impairment, Parkinson’s disease-like mitochondrial dysfunction) [1-5] - Its low toxicity (no obvious肝肾毒性 in animal models) and natural origin make it a promising candidate for functional food or pharmaceutical development [1,4] ; |
| 分子式 |
C27H32O14
|
|
|---|---|---|
| 分子量 |
580.53
|
|
| 精确质量 |
580.179
|
|
| CAS号 |
10236-47-2
|
|
| 相关CAS号 |
Naringin Dihydrochalcone;18916-17-1
|
|
| PubChem CID |
442428
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
928.1±65.0 °C at 760 mmHg
|
|
| 熔点 |
166 °C
|
|
| 闪点 |
308.5±27.8 °C
|
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
|
| 折射率 |
1.564
|
|
| LogP |
-0.18
|
|
| tPSA |
225.06
|
|
| 氢键供体(HBD)数目 |
8
|
|
| 氢键受体(HBA)数目 |
14
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
41
|
|
| 分子复杂度/Complexity |
884
|
|
| 定义原子立体中心数目 |
11
|
|
| SMILES |
C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)O[C@@H]2[C@H]([C@@H]([C@H](O[C@H]2OC3=CC(=C4C(=O)C[C@H](OC4=C3)C5=CC=C(C=C5)O)O)CO)O)O)O)O)O
|
|
| InChi Key |
DFPMSGMNTNDNHN-JJLSSNRUSA-N
|
|
| InChi Code |
InChI=1S/C27H32O14/c1-10-20(32)22(34)24(36)26(37-10)41-25-23(35)21(33)18(9-28)40-27(25)38-13-6-14(30)19-15(31)8-16(39-17(19)7-13)11-2-4-12(29)5-3-11/h2-7,10,16,18,20-30,32-36H,8-9H2,1H3/t10-,16?,18+,20-,21+,22+,23-,24+,25+,26-,27+/m0/s1
|
|
| 化学名 |
7-(((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-5-hydroxy-2-(4-hydroxyphenyl)chroman-4-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (3.58 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (3.58 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.58 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 40 mg/mL (68.90 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7226 mL | 8.6128 mL | 17.2256 mL | |
| 5 mM | 0.3445 mL | 1.7226 mL | 3.4451 mL | |
| 10 mM | 0.1723 mL | 0.8613 mL | 1.7226 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01272167 | Completed | Behavioral: Long-term grapefruit juice consumption |
Post-menopausal Status | University Hospital, Clermont-Ferrand | March 2010 | Not Applicable |
| NCT01423019 | Completed | Dietary Supplement: Advantra Z + Naringin + Hesperiden |
Weight Loss | Integrative Health Technologies, Inc. | October 2011 | Not Applicable |
| NCT03582553 | Completed Has Results | Dietary Supplement: Naringenin Other: Placebo |
Safety Issues Pharmacokinetics |
Pennington Biomedical Research Center | May 25, 2018 | Early Phase 1 |
| NCT03928249 | Completed | Dietary Supplement: Eriocitrin | Pre Diabetes | São Paulo State University | December 1, 2019 | Not Applicable |
|
|
|