| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
γ-secretase (IC50 = 6.2 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:在使用 HPB-ALL 细胞进行的细胞测定中,PF-03084014 抑制 Notch 受体裂解,这些细胞在 Notch1 的异二聚化和 PEST 结构域中均存在突变,IC50 为 13.3 nM。 PF-03084014 下调 HPB-ALL 细胞中 Notch 靶基因 Hes-1 和 cMyc 的表达,IC50 分别<1 nM 和 10 nM。 PF-03084014 通过诱导细胞周期停滞和细胞凋亡来抑制人类 T-ALL 细胞系子集(HPB-ALL、DND-41、TALL-1 和 Sup-T1)的细胞生长,IC50 为 30-100 nM。 PF-03084014 可减少 HUVEC 的增殖,IC50 为 0.5 μM,并减少管腔形成,IC50 值为 50 nM。 PF-03084014 (1 μM) 对 MX1 细胞没有抗增殖作用;然而,它可以抑制 95% 的迁移。激酶测定:使用适当设计的寡核苷酸和APP695 cDNA通过PCR扩增产生编码APP (APP695)的695-aa同工型的氨基酸596-695和C末端的标志序列(DYKDDDDK)的DNA片段。充当翻译起始位点的Met是APP695的残基596(相对于β-分泌酶切割位点的P1残基)。将该DNA片段插入原核表达载体pET2-21b中。重组蛋白 C100Flag 在大肠杆菌 [菌株 BL21(DE3)] 中过量产生,并通过 Mono-Q 柱层析纯化。在 CHAPSO、CHAPS(3-[(3-胆酰胺丙基)二甲基氨基]-1-丙磺酸盐)或 Triton X-100(0、 0.125、0.25、0.5 或 1%),溶于缓冲液 B(50 mM 管道,pH 7.0y 5mM MgCl2/5 mM CaCl2/150 mM KCl),37°C。通过添加 RIPA(150 mM NaCl/1.0% NP-40/0.5% 脱氧胆酸钠 0.1% SDS/50 mM Tris HCl,pH 8.0)并煮沸 5 分钟来终止反应。将样品离心并通过ECL测定上清液中的Aβ肽。来自 γ 分泌酶介导的 C100Flag 加工的 Aβ40 和 Aβ42 相关产物在 N 末端具有 Met,因此分别定义为 M-Aβ40 和 M-Aβ42。同样,将 CHAPSO 提取的 HeLa 细胞膜(溶解的 γ-分泌酶)的上清液 (0.125 mg/mL) 与含有 0.25% CHAPSO 的缓冲液 B 中的 C100Flag (1.7 μM) 一起孵育,随后通过以下方法测定 M-Aβ40 和 M-Aβ42:使用ECL。细胞测定:将细胞(人 T-ALL 细胞系 HPB-ALL)以 10,000 个细胞/孔接种在 96 孔板中,生长培养基中补充有 10% 胎牛血清。在DMSO中连续稀释PF-03084014,向每孔中添加适当的对照或指定浓度的PF-03084014,并将细胞在37℃下孵育7天(最终DMSO含量0.1%)。将终浓度为 0.1 mg/mL 的刃天青添加到细胞中,并将板孵育 2 至 4 小时。荧光信号读取为 560 nm 激发后 590 nm 处的发射。
|
| 体内研究 (In Vivo) |
PF-03084014 单剂量口服 200 mg/kg,可在异种移植 HPB-ALL 肿瘤中产生约 80% 的最大 NICD 抑制。 PF-03084014 在这种模式下显示出强大的抗肿瘤活性,在 150 mg/kg 的剂量下,最大肿瘤生长抑制率为 92%,同时 NICD/Notch1、肿瘤有丝分裂指数 (Ki67) 和细胞凋亡(激活的 caspase)显着降低-3)染色。 PF-03084014 (120 mg/kg) 可诱导乳腺癌 HCC1599 荷瘤小鼠凋亡、抗增殖、降低肿瘤细胞自我更新能力、损害肿瘤脉管系统并降低转移活性。 PF-03084014治疗在各种类型的乳腺异种移植模型中显示出显着的抗肿瘤活性,TGI值至少为50%。
|
| 酶活实验 |
通过使用适当制作的寡核苷酸和 APP695 cDNA 进行 PCR 扩增,产生了编码 APP (APP695) 695-aa 同种型的氨基酸 596-695 和 C 末端标记序列 (DYKDDDDK) 的 DNA 片段。 APP695 的其余部分 596,或与 β-分泌酶切割位点相关的 P1 残基,是充当翻译起始位点的 Met。细菌表达载体 pET2-21b 中插入了该 DNA 片段。重组蛋白 C100Flag 在大肠杆菌 [菌株 BL21(DE3)] 中过量生产后,使用 Mono-Q 柱层析进行纯化。 C100Flag (1.7 μM) 与细胞膜 (0.5 mg/mL) 在缓冲液 B(50 mM 管道,pH 7.0y 5mM MgCl2/5 mM CaCl2/ 150 mM KCl),37°C,CHAPSO、CHAPS(3-[(3-胆酰胺丙基)二甲基氨基]-1-丙磺酸盐)或 Triton X-100(0、0.125、0.25、0.5 或 1)存在下%) 或在 37°C 下孵育。添加 RIPA(150 mM NaCl/1.0% NP-40/0.5% 脱氧胆酸钠 0.1% SDS/50 mM Tris HCl,pH 8.0)并煮沸五分钟以停止反应。离心样品后,使用 ECL 测试上清液以检测 Aβ 肽的存在。由γ-分泌酶加工C100Flag产生的产物Aβ40和Aβ42在其N末端具有Met,这分别使它们成为M-Aβ40和M-Aβ42。类似地,将用 CHAPSO(溶解的 γ-分泌酶)提取的 HeLa 细胞膜的 0.125 mg/mL 上清液与含有 0.25% CHAPSO 的缓冲液 B 中的 C100Flag (1.7 μM) 一起孵育。随后进行 M-Aβ40 和 M-Aβ42 的 ECL 测定。
|
| 细胞实验 |
使用补充有 10% 胎牛血清的生长培养基在 96 孔板中以 10,000 个细胞/孔接种。将PF-03084014在DMSO中连续稀释,向每个孔中添加一定浓度的化合物或合适的对照,并将细胞在37°C下孵育7天(最终DMSO含量为0.1%)。用终浓度为 0.1 mg/mL 的刃天青处理细胞后,将板孵育 2 至 4 小时。在 560 nm 激发后,在 590 nm 处发射,这是测量荧光信号的方式。
|
| 动物实验 |
Mice: Female athymic mice (nu/nu, 6-8 weeks) are employed. Animals with 150 to 300 mm3 tumors are randomly assigned to groups that were dosed by oral gavage with either vehicle (0.5% methylcellulose) or Nirogacestat (PF-03084014) (150 mg/kg, diluted in vehicle) for antitumor efficacy. Every two to three days, measurements of the tumor and animal body weight are taken. Vernier calipers are used to measure and calculate tumor volume (mm3). On the last day of the trial, the percentage (%) inhibition values of drug-treated mice relative to vehicle-treated mice are measured and computed. Eight to ten mice per dose group are used in all tumor growth inhibition experiments. To ascertain the P value, the student's t test is employed.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The following pharmacokinetics parameters in patients with desmoid tumors were calculated as follows: Cmax (508 (62) ng/mL), AUC0-tau (u 3370 (58) ng·h/mL), time to steady state (6 days), and Tmax (1.5 (0.5, 6.5) hours). Nirogacestat is excreted mostly in feces (38%) and urine 17%, with less than 1% of the unchanged drug remains in the urine. It can also be eliminated through expired air (9.7%). The apparent volume of distribution [Mean (%CV)] of nirogacestat is 1430 (65) L. The apparent systemic clearance [Mean (%CV)] of nirogacestat is 45 (58) L/hr. Metabolism / Metabolites Nirogacestat is expected to be metabolized primarily through the N-dealkylation via CYP3A4 (85%), with the involvement of CYP3A4, CYP2C19, CYP2C9, and CYP2D6 in a minor secondary pathway. Biological Half-Life The terminal elimination half-life [Mean (%CV)] of nirogacestat is 23 (37) hr . |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Nirogacestat has a high level of serum protein binding of 99.6%, with 94.6% to serum albumin and 97.9% to alpha-1 acid glycoprotein. |
| 参考文献 | |
| 其他信息 |
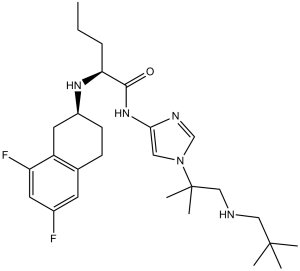
Nirogacestat is a member of the class of imidazoles that is 1H-imidazole substituted by a 1-[(2,2-dimethylpropyl)amino]-2-methylpropan-2-yl group at position 1 and a {N-[(2S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl]-L-norvalyl}amino group at position 4. It is a gamma-secretase inhibitor whose hydrobromide salt is indicated for adult patients with progressing desmoid tumours who require systemic treatment. It has a role as an antineoplastic agent and a gamma-secretase modulator. It is a member of tetralins, an organofluorine compound, a secondary carboxamide, a secondary amino compound and a member of imidazoles. It is a conjugate base of a nirogacestat(2+).
Nirogacestat is a small-molecule gamma-secretase inhibitor that was investigated as a potential treatment for desmoid tumors. Desmoid tumors are typically characterized by aberrant activation in Notch signaling. Interaction between the notch receptors and their ligands activates proteolytic cleavage by gamma-secretase; therefore, the inhibition of gamma-secretase can potentially inhibit Notch signaling and thus impede the growth of desmoid tumors. Nirogacestat was approved under the brand name OGSIVEO on November 27, 2023, by the FDA for the treatment of adult patients with progressing desmoid tumors who require systemic treatment. It was previously granted breakthrough therapy, fast track, and orphan drug designations for the treatment of desmoid tumors, and the final approval was based on positive results obtained in the Phase 3 DeFi trial, where a confirmed objective response rate was observed to be 41% compared to 8% with the placebo. Nirogacestat is a selective gamma secretase (GS) inhibitor with antitumor activity. Upon administration, nirogacestat targets and binds to GS, thereby blocking the proteolytic activation of Notch receptors. This inhibits the Notch signaling pathway and results in the induction of apoptosis in tumor cells that overexpress Notch. The integral membrane protein GS is a multi-subunit protease complex that cleaves single-pass transmembrane proteins, such as Notch receptors, at residues within their transmembrane domains. Overexpression of the Notch signaling pathway has been correlated with increased tumor cell growth and survival. Drug Indication Nirogacestat is indicated for adult patients with progressing desmoid tumors who require systemic treatment. Mechanism of Action Nirogacestat is a gamma secretase inhibitor that blocks proteolytic activation of the Notch receptor. When dysregulated, Notch can activate pathways that contribute to tumor growth. Pharmacodynamics There is an exposure-response relationship between nirogacestat exposure and Grade 3 hypophosphatemia with a higher risk of Grade 3 hypophosphatemia at higher exposure. At the recommended dosage, a mean increase in the QTc interval > 20 ms was not observed. |
| 分子式 |
C27H41F2N5O
|
|
|---|---|---|
| 分子量 |
489.64
|
|
| 精确质量 |
489.328
|
|
| 元素分析 |
C, 66.23; H, 8.44; F, 7.76; N, 14.30; O, 3.27
|
|
| CAS号 |
1290543-63-3
|
|
| 相关CAS号 |
Nirogacestat dihydrobromide;1962925-29-6
|
|
| PubChem CID |
46224413
|
|
| 外观&性状 |
White to yellow solid powder
|
|
| LogP |
6.217
|
|
| tPSA |
74.47
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
11
|
|
| 重原子数目 |
35
|
|
| 分子复杂度/Complexity |
685
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
FC1=C([H])C(=C([H])C2=C1C([H])([H])[C@]([H])(C([H])([H])C2([H])[H])N([H])[C@]([H])(C(N([H])C1=C([H])N(C([H])=N1)C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])N([H])C([H])([H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])=O)C([H])([H])C([H])([H])C([H])([H])[H])F
|
|
| InChi Key |
VFCRKLWBYMDAED-REWPJTCUSA-N
|
|
| InChi Code |
InChI=1S/C27H41F2N5O/c1-7-8-23(32-20-10-9-18-11-19(28)12-22(29)21(18)13-20)25(35)33-24-14-34(17-31-24)27(5,6)16-30-15-26(2,3)4/h11-12,14,17,20,23,30,32H,7-10,13,15-16H2,1-6H3,(H,33,35)/t20-,23-/m0/s1
|
|
| 化学名 |
(2S)-2-[[(2S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl]amino]-N-[1-[1-(2,2-dimethylpropylamino)-2-methylpropan-2-yl]imidazol-4-yl]pentanamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (5.11 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声和加热处理
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (5.11 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.11 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1.43 mg/mL (2.92 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: 5% DMSO+40% PEG 300+5%Tween80+ 50%ddH2O: 2.4mg/ml 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0423 mL | 10.2116 mL | 20.4232 mL | |
| 5 mM | 0.4085 mL | 2.0423 mL | 4.0846 mL | |
| 10 mM | 0.2042 mL | 1.0212 mL | 2.0423 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05949099 | Recruiting | Drug: Nirogacestat Procedure: Cryoablation |
Desmoid Tumor | Stanford University | August 15, 2023 | Phase 2 |
| NCT05348356 | Active Recruiting |
Drug: Nirogacestat | Ovarian Granulosa Cell Tumor Ovarian Cancer |
Medanta, The Medicity, India | August 30, 2022 | Phase 2 |
| NCT05879146 | Not yet recruiting | Drug: Nirogacestat | Tumor | M.D. Anderson Cancer Center | October 30, 2023 | Phase 2 |
| NCT05556798 | Recruiting | Drug: Nirogacestat Drug: Pomalidomide |
Multiple Myeloma | Memorial Sloan Kettering Cancer Center |
October 4, 2022 | Phase 1 |
| NCT03785964 | Active Recruiting |
Drug: Nirogacestat oral tablet Drug: Placebo Oral Tablet |
Aggressive Fibromatosis Desmoid Tumor |
SpringWorks Therapeutics, Inc. | April 17, 2019 | Phase 3 |
|
|---|
|
|
|
|---|
|