| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
EGFRL858R (IC50 = 12 nM); EGFRL858R/T790M (IC50 = 1 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:与野生型体外相比,AZD9291 对突变型 EGFR 细胞系的增殖具有明显更有效的抑制作用。激酶测定:Osimertinib(以前称为 AZD-9291 和 mereletinib)是一种口服的、不可逆的、突变选择性的第三代 EGFR 抑制剂,对于外显子 19 缺失 EGFR、L858R/T790M EGFR、IC50 分别为 12.92、11.44 和 493.8 nM。和 WT EGFR 分别在 LoVo 细胞中。它抑制激活性和耐药性 EGFR 突变,同时保留正常皮肤和肠道细胞中存在的正常形式 EGFR,从而减少当前可用药物遇到的副作用。细胞测定:AZD9291 在体外有效抑制 EGFRm+(例如 PC9;< 25 nM)和 EGFR m+/T790M(例如 H1975;< 25 nM)细胞系中的 EGFR 磷酸化,同时对野生型 EGFR 系(例如 LoVo)的活性要低得多;> 500 nM)。一致地,与野生型体外相比,AZD9291 在突变型 EGFR 细胞系中显示出更有效的增殖抑制作用。
|
||
| 体内研究 (In Vivo) |
AZD9291(5mg/kg po)可引起 EGFRm+ (PC9) 和 EGFRm+/T790M (H1975) 肿瘤模型的肿瘤深度消退,并在体内深度抑制 EGFR 磷酸化和关键下游信号通路(如 AKT 和 ERK)。
|
||
| 酶活实验 |
在康宁黑色透明底 384 孔板中,每孔在生长培养基中接种 10,000 个细胞,然后将板在 37°C、5% CO2 下孵育整晚。将化合物在 100% DMSO 中连续稀释,并使用 Echo 555 对细胞进行声学给药。吸出培养基并将板再孵育两小时后,向每孔中添加 40μL 裂解缓冲液。 Greiner black 高结合 384 孔板在包被捕获抗体后用 3% BSA 封闭。去除块后,将15μL裂解液添加至Greiner black高结合384孔板中,并将板孵育2小时。添加检测抗体(20μL),并在抽吸和PBS洗涤后将板孵育两小时。抽吸和 PBS 洗涤后添加 20μL QuantaBlu 荧光过氧化物酶底物并孵育 1 小时。在每个板中添加 20μL QuantaBlu 终止液,并使用 Envision 读板器读取 352 nm(激发)和 460 nm(发射)的荧光。使用合适的软件程序导出每种化合物获得的数据并进行曲线拟合分析。通过计算产生 50% 效果所需的化合物浓度,从该数据获得 IC50 值。
|
||
| 细胞实验 |
在体外,AZD9291 在 EGFRm+(例如 PC9;< 25 nM)和 EGFR m+/T790M(例如 H1975;< 25 nM)细胞系中显示出 EGFR 磷酸化的显着抑制作用,但对野生型 EGFR 细胞系(例如,LoVo;> 500 nM)。在体外,与野生型相比,AZD9291 在突变型 EGFR 细胞系中持续表现出更强大的增殖抑制作用。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The median time to Cmax was found to be 6 hours. Osimertinib is primarily eliminated through excretion in the feces (68%), to a lesser extent through urine (14%), while only 2% is excreted unchanged. The mean volume of distribution at steady state is 918 L. Oral clearance is 14.3 L/hr. Metabolism / Metabolites Osimertinib is metabolized to at least two pharmacologically active metabolites, AZ7550 and AZ5104, that circulate at approximately 10% of the concentration of the parent compound. Biochemical assays have shown that AZ7550 has similar potency and efficacy to osimertinib, while AZ5104 is more potent against mutant and wild-type EGFR. The main metabolic pathways are oxidation (predominantly by CYP3A) and dealkylation. Biological Half-Life The population estimated mean half-life is 48 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elevations in serum aminotransferase levels are uncommon during osimertinib therapy occurring in 4% to 5% of patients and rising above 5 times the upper limit of the normal range in only 1% or less. In preregistration trials, there was a single incidence of clinically apparent liver injury attributed to osimertinib therapy, but the clinical features and relatedness to therapy were not defined. Since its approval and more widespread use, there have been no published cases of liver injury due to osimertinib. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of osimertinib during breastfeeding. Because osimertinib is 95% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 48 hours and it might accumulate in the infant. The drug also has 2 active metabolites that have not been studied in breastmilk. The manufacturer recommends that breastfeeding be discontinued during osimertinib therapy and for 2 weeks after the final dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding of osimertinib is 95%. |
||
| 参考文献 |
[1]. Patent. 2013, WO2013014448 A1. [2]. Mol Cancer Ther (2013) 12 (11_Supplement): A109. [3]. Sci Transl Med. 2022 Mar 30;14(638):eabc7480. [4]. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014 Sep;4(9):1046-61.[5]. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistancemutations that spares the wild type form of the receptor. J Med Chem. 2014 Oct 23;57(20):8249-67. |
||
| 其他信息 |
Pharmacodynamics
A pharmacokinetic/pharmacodynamic analysis suggested a concentration-dependent QTc interval prolongation of 14 msec (upper bound of two-sided 90% CI: 16 msec) at a dose of osimertinib 80 mg. |
| 分子式 |
C28H33N7O2
|
|---|---|
| 分子量 |
499.61
|
| 精确质量 |
499.269
|
| 元素分析 |
C, 67.31; H, 6.66; N, 19.62; O, 6.40
|
| CAS号 |
1421373-65-0
|
| 相关CAS号 |
Osimertinib mesylate;1421373-66-1;Osimertinib-d6;1638281-44-3;Osimertinib dimesylate;2070014-82-1;Osimertinib-13C,d3;2254100-49-5
|
| PubChem CID |
71496458
|
| 外观&性状 |
Brown to yellow solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 折射率 |
1.618
|
| LogP |
3.3
|
| tPSA |
87.55
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
752
|
| 定义原子立体中心数目 |
0
|
| SMILES |
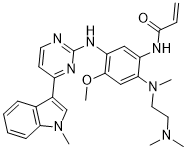
O(C([H])([H])[H])C1=C(C([H])=C(C(=C1[H])N(C([H])([H])[H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H])N([H])C(C([H])=C([H])[H])=O)N([H])C1=NC([H])=C([H])C(C2=C([H])N(C([H])([H])[H])C3=C([H])C([H])=C([H])C([H])=C32)=N1
|
| InChi Key |
DUYJMQONPNNFPI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32)
|
| 化学名 |
N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide
|
| 别名 |
AZD-9291; AZD9291; AZD 9291; Mereletinib; AZD9291; AZD 9291; UNII-3C06JJ0Z2O; Osimertinib [USAN]; Osimertinib free base; Mereletinib; Trade name: Tagrisso
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.00 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.00 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.16 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% DMSO+30% PEG 300+dd H2O: 30mg/mL 配方 5 中的溶解度: 5 mg/mL (10.01 mM) in 0.5%HPMC 1%Tween80 (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶。 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0016 mL | 10.0078 mL | 20.0156 mL | |
| 5 mM | 0.4003 mL | 2.0016 mL | 4.0031 mL | |
| 10 mM | 0.2002 mL | 1.0008 mL | 2.0016 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study of Precision Treatment for Rare Tumours in China Guided by PDO and NGS
CTID: NCT06692491
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-11-18
AZD9291 binding mode and structure.Cancer Discov.2014 Sep;4(9):1046-61. |
Effect of AZD9291 on EGFR phosphorylationin vitro.Cancer Discov.2014 Sep;4(9):1046-61. |
In vivoanti-tumor efficacy of AZD9291 in subcutaneous xenograft models of EGFR-TKI sensitising and T790M resistant lung cancer.Cancer Discov.2014 Sep;4(9):1046-61. |
AZD9291 induces significant and sustained tumor regression in transgenic models of EGFR-TKI sensitising (C/L858R) and T790M resistant (C/L+T) lung cancer.Cancer Discov.2014 Sep;4(9):1046-61. |
AZD9291 inhibits EGFR phosphorylation and downstream signallng in murine models of EGFR T790M resistant lung cancer.Cancer Discov.2014 Sep;4(9):1046-61. |
Proof of concept clinical studies validating AZD9291 as a mutant-selective EGFR kinase T790M inhibitor.Cancer Discov.2014 Sep;4(9):1046-61. |