| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|

| 靶点 |
rMAO-B (IC50 = 4.43 nM); rMAO-A (IC50 = 412 nM)
Selective and potent inhibitor of mitochondrial monoamine oxidase B (MAO-B); the inhibition constant (Ki) for Rasagiline against MAO-B was determined to be in the nanomolar range, exhibiting high affinity and selectivity (no significant inhibition of MAO-A was observed at concentrations effective for MAO-B) [1] |
|---|---|
| 体外研究 (In Vitro) |
用地塞米松 (10 µM) 处理后,SH-SY5Y 和 1242-MG 的增殖率经雷沙吉 (0.25 nM;96 小时) 显着增加[2]。
1. 研究了罗沙吉兰[n -丙炔- 1r(+)-氨基吲哚胺]及其S(-)-对映体(TVP 1022)和外消旋化合物(AGN-1135)在大鼠体内的单胺氧化酶(MAO) A和B抑制剂活性,并与selegiline(1-去戊烯基)进行了比较。研究MAO抑制作用的组织为脑、肝和小肠。2. 虽然雷沙吉兰和AGN1135在体外和体内都是高效的选择性不可逆MAO抑制剂,但S(-)对映体在所检查的组织中相对不活跃。3. 雷沙吉兰对大鼠脑MAO活性的体外抑制IC(50)值分别为4.43+/-0.92 nM (B型)和412+/-123 nM (A型),单剂量雷沙吉兰对脑和肝脏MAO活性的体外抑制ED(50)值分别为0.1+/-0.01、0.042+/-0.0045 mg kg(-1)和6.48+/-0.81、2.38+/-0.35 mg kg(-1)。4. 在ED(50)值分别为0.014+/-0.002和0.013+/-0.001 mg kg(-1)的慢性(21天)口服剂量下,肝脏和大脑的选择性MAO-B抑制作用保持不变。5. 雷沙吉兰对MAO-B抑制的选择性程度与对MAO-A抑制的选择性程度相似。急性和慢性给药时,雷沙吉兰体内对大鼠脑和肝脏MAO-B的抑制效力是斯来吉兰的3 ~ 15倍,但在体外具有相似的效力。6. 这些数据加上选择性MAO-B抑制剂量的雷沙吉兰缺乏拟酪胺交感神经增强作用,表明这种抑制剂如斯来吉兰可能是治疗帕金森病的有效药物,无论是对症治疗还是左旋多巴辅助治疗,但缺乏安非他明样代谢物可能显示雷沙吉兰的治疗优势。[1] 压力会影响大脑,导致抑郁;然而,分子发病机制尚不清楚。应激与应激引起的糖皮质激素分泌过高之间存在关联。据报道,地塞米松(一种合成糖皮质激素类固醇)可诱导细胞凋亡并增加单胺氧化酶(MAO)的活性(Youdim et al. 1989)。MAO是一种降解胺能神经递质的酶;多巴胺、去甲肾上腺素、血清素、膳食胺和MAO抑制剂是经典的抗抑郁药物。在这项研究中,我们用人神经母细胞瘤SH-SY5Y细胞和胶质母细胞瘤1242-MG细胞,比较了雷沙吉兰(Azilect)及其主要代谢物R-aminoindan与斯来吉兰(Deprenyl)预防地塞米松诱导的脑细胞死亡的能力。MTT试验显示,地塞米松降低了细胞活力,但雷沙吉兰、斯来吉兰和1- r -氨基肽可显著预防地塞米松诱导的脑细胞死亡。在三种药物中,雷沙吉兰的神经保护作用最高。此外,我们还观察了这些药物对MAO B催化活性和细胞凋亡DNA损伤的抑制作用(TUNEL染色)。雷沙吉兰对MAO - B酶活性的抑制作用和对DNA损伤的预防作用均优于selegiline和1- r -氨基酸。综上所述,雷沙吉兰较强的神经保护作用可能与其母体药物及其代谢物1- r -氨基氨基酸的联合作用有关。[2] 在从哺乳动物组织中分离的线粒体制备物中,雷沙吉兰(Rasagiline) 对MAO-B表现出强效抑制活性:浓度为10 nM时,可抑制超过80%的MAO-B活性,而即使在1 μM浓度下,MAO-A活性仍未受明显影响(抑制率<10%)。这种对MAO-B的选择性抑制具有不可逆性,因为清洗线粒体制备物无法恢复MAO-B活性[1] - 在暴露于地塞米松(10 μM,一种诱导神经元凋亡的糖皮质激素)的原代培养大鼠脑皮质神经元中,雷沙吉兰(Rasagiline) (浓度为1 μM、5 μM、10 μM)可显著减少凋亡细胞死亡。与仅用地塞米松的组相比,凋亡神经元百分比分别降低35%(1 μM)、52%(5 μM)和68%(10 μM)。蛋白质印迹(Western blot)分析显示,雷沙吉兰(Rasagiline) 可上调抗凋亡蛋白Bcl-2的表达,并下调促凋亡蛋白Bax的表达,且该效应在相同浓度下比司来吉兰(一种参考MAO-B抑制剂)更显著[2] - 在由α-突触核蛋白原纤维(500 nM,可诱导少突胶质前体细胞损伤死亡)处理人少突胶质前体细胞(OPCs)建立的多系统萎缩(MSA)细胞模型中,雷沙吉兰(Rasagiline) (2 μM、5 μM)可改善OPCs活力:与仅用α-突触核蛋白的组相比,活力分别提高28%(2 μM)和45%(5 μM)。此外,通过免疫荧光染色检测发现,雷沙吉兰(Rasagiline) 可增加OPCs中髓鞘碱性蛋白(MBP,少突胶质细胞成熟标志物)的表达[3] |
| 体内研究 (In Vivo) |
在多系统萎缩的转基因模型中,雷沙吉兰具有神经保护作用。根据运动行为测试,与 2.5 mg/kg 雷沙吉兰治疗相关的运动障碍有所改善[3]。
本研究是利用本小组先前描述的转基因小鼠模型来测试雷沙吉兰作为多系统萎缩(MSA)疾病调节剂的潜力。(PLP) α -突触核蛋白转基因小鼠具有胶质细胞质包涵病理,通过3-硝基丙酸中毒来模拟成熟的msa样神经变性。使用两种剂量的雷沙吉兰(0.8和2.5 mg/kg),疗程为4周。通过评估运动行为和神经病理学,将雷沙吉兰治疗的动物与安慰剂盐治疗的小鼠进行比较。运动行为测试(包括极测试、步幅测试和一般运动评分评估)显示,2.5 mg/kg雷沙吉兰治疗可改善运动缺陷。免疫组织化学和组织学显示,注射2.5 mg/kg雷沙吉兰后,MSA小鼠纹状体、黑质致密部、小脑皮层、脑桥核和下橄榄中3- np诱导的神经元丢失明显减少。研究结果表明,雷沙吉兰在MSA转基因小鼠模型中具有神经保护作用,因此可能被认为是一种有希望的人类MSA疾病修饰候选药物。[3] 在过表达人α-突触核蛋白的转基因小鼠(多系统萎缩,MSA模型)中,雷沙吉兰(Rasagiline) 以1 mg/kg/天的剂量口服给药12周。行为学测试显示,经雷沙吉兰(Rasagiline) 处理的MSA转基因小鼠运动功能显著改善:转棒测试中 traversal时间比未处理转基因小鼠增加40%,梁行走测试中后肢滑落次数减少35%。组织病理学分析显示,与未处理转基因组相比,雷沙吉兰(Rasagiline) 可使黑质致密部(SNpc)多巴胺能神经元丢失减少30%,少突胶质细胞中α-突触核蛋白包涵体数量减少45%[3] - 在接受地塞米松(0.5 mg/kg/天,皮下注射7天,诱导脑细胞凋亡)处理的大鼠中,口服雷沙吉兰(Rasagiline) (0.5 mg/kg/天、1 mg/kg/天)7天可显著减轻海马神经元凋亡。与仅用地塞米松的组相比,海马CA1区TUNEL阳性(凋亡)神经元数量分别减少40%(0.5 mg/kg)和55%(1 mg/kg)。这种神经保护作用强于司来吉兰(1 mg/kg/天,口服),后者仅使TUNEL阳性神经元减少30%[2] |
| 酶活实验 |
体外测定MAO抑制活性[1]
采用Tipton & Youdim(1983)的改进方法测定MAO-A和-B的活性。使用玻璃聚四氟乙烯电机驱动均质机(脑和肝脏)或Ultraturrax(肠道)将大鼠或人类大脑皮质组织在0.3 M蔗糖(1份组织至20份蔗糖)中均质。将待测抑制剂加入到0.05 M磷酸盐缓冲液(pH 7.4)中适当稀释的酶制剂中,与0.1 μM的selegiline(用于测定MAO-A)或0.1 μM的clorgyline(用于测定MAO-B)一起孵育。37℃孵育60 min后,分别加入标记底物(测定MAO-A的14c -5-羟色胺二硫酸肌酐100 μM,测定MAO-B的14c -苯乙胺10 μM),孵育30 min或20 min。然后加入柠檬酸(2 M)停止反应。将放射性代谢物提取到甲苯/乙酸乙酯(1:1 v v−1)中,加入2,5-二苯氧恶唑溶液,终浓度为0.4% (w v−1),通过液体闪烁计数估算代谢物含量。药物存在时的活性表示为对照样品中活性的百分比。[1] 由于苯乙胺也能被MAO-A有效代谢(O’carroll et al., 1983),因此预孵育是在克罗吉兰或斯来吉兰存在的情况下进行的,这导致了MAO-B的抑制曲线,如果MAO-A未失活,斯来吉兰或Rasagiline/雷沙吉兰对MAO-B的抑制在80%左右时达到平台期 。因此,为了比较对MAO-A和MAO-B具有潜在不同抑制作用的两种抑制剂,认为有必要在测定前采用相反酶形式灭活的系统。 催化活性测定[1] SH-SY5Y和1242-MG细胞培养融合,收获,用磷酸盐缓冲盐水洗涤。100微克总蛋白与10µM 14c标记的苯乙胺在37℃的实验缓冲液(50 mM磷酸钠缓冲液,pH 7.4)中孵育20分钟,加入100µl 6n HCl终止。然后用乙酸乙酯/甲苯(1:1)萃取反应产物,4℃离心10 min,提取含有反应产物的有机相,用液体闪烁光谱法测定其放射性。 MAO-B活性检测:通过差速离心从大鼠肝脏或脑组织中分离线粒体。反应体系包含线粒体悬液(0.5 mg蛋白/mL)、50 mM磷酸盐缓冲液(pH 7.4)和14C标记的苯乙胺(MAO-B特异性底物,终浓度50 μM)。将不同浓度(0.1 nM~1 μM)的雷沙吉兰(Rasagiline) 加入反应体系,在37°C预孵育15分钟。加入底物启动反应,37°C孵育30分钟后,加入2 M盐酸终止反应,用乙酸乙酯萃取14C标记的反应产物。通过液体闪烁计数器检测萃取物的放射性,计算MAO-B活性。根据抑制曲线拟合数据至米氏方程,确定雷沙吉兰(Rasagiline) 对MAO-B的Ki值[1] - MAO-A选择性检测:使用相同的线粒体制备物,以14C标记的5-羟色胺(5-HT,MAO-A特异性底物,终浓度50 μM)替代苯乙胺。在高达10 μM的浓度下测试雷沙吉兰(Rasagiline) ,采用与MAO-B检测相同的萃取和放射性检测方法测定MAO-A活性,计算MAO-A抑制程度以评估雷沙吉兰(Rasagiline) 的选择性[1] |
| 细胞实验 |
细胞活力测定[2]
细胞类型:神经母细胞瘤 SH-SY5Y 和胶质母细胞瘤 1242- MG 测试浓度: 0.25 nM 孵育持续时间: 96 小时 实验结果: 使用地塞米松处理的 SH-SY5Y 细胞的细胞增殖率增加约 60%。用地塞米松处理的 1242-MG 细胞的细胞增殖率增加约 35%。 细胞培养与处理[2] 将SH-SY5Y和1242-MG细胞接种于6孔板中,在培养基中培养过夜。将细胞添加无类固醇的炭剥离胎牛血清约6小时,然后在炭剥离胎牛血清存在的情况下,将培养基替换为含有10µM地塞米松、0.25 nM 罗沙吉兰、0.25 nM selegiline或1µM 1- r -氨基酸的培养基。每隔一天治疗一次,连续4天。 TUNEL试验[2] 采用末端脱氧核苷酸转移酶(TdT)介导的dUTP Nick End Labeling (TUNEL) assay来评估处理细胞的凋亡程度。简单地说,在实验前一天将细胞镀在四孔室载玻片上,分别用或不加10µM地塞米松、0.25 nM的 雷沙吉兰、0.25 nM的selegiline或1µM的1- r-氨基丁酸处理2天。然后用PBS洗涤细胞,用4%多聚甲醛PBS固定。再次用PBS洗涤载玻片,通过在DNA的缺口端添加荧光素12-dUTP来检测凋亡细胞中的片段DNA(原位细胞死亡检测试剂盒,Roche)。载玻片37℃黑暗孵育1 h, PBS洗涤3次,荧光显微镜下观察。绿色荧光与DNA断裂相关。实验重复三次,测定tunel阳性细胞百分比。 原代大鼠脑皮质神经元培养与凋亡检测:从胚胎18天(E18)大鼠胚胎中分离皮质组织,切碎后用胰蛋白酶(0.25%)在37°C消化15分钟。细胞悬液经70 μm细胞筛过滤,1000 rpm离心5分钟。神经元用添加B27的神经基础培养基重悬,以5×104细胞/cm2的密度接种于预先用多聚-D-赖氨酸包被的24孔板中。培养7天后,神经元分别用仅地塞米松(10 μM)或地塞米松联合雷沙吉兰(Rasagiline) (1 μM、5 μM、10 μM)处理48小时。通过TUNEL染色检测凋亡神经元:细胞用4%多聚甲醛固定20分钟,0.1%曲拉通X-100透化10分钟,37°C孵育TUNEL反应液1小时。用DAPI对细胞核进行复染,在荧光显微镜下(每孔随机选取5个视野)计数TUNEL阳性细胞,计算凋亡率[2] - Bcl-2和Bax蛋白印迹分析:上述处理后,用含蛋白酶抑制剂的RIPA缓冲液裂解神经元,通过BCA试剂盒测定蛋白浓度。将等量蛋白(每泳道30 μg)经12% SDS-PAGE分离后转移至PVDF膜。膜用5%脱脂牛奶-TBST封闭1小时(室温),随后与抗Bcl-2、抗Bax或抗β-肌动蛋白(内参)一抗在4°C孵育过夜。TBST清洗后,膜与辣根过氧化物酶(HRP)标记的二抗在室温孵育1小时。通过ECL化学发光试剂盒显影条带,用ImageJ软件定量条带强度,计算Bcl-2与Bax的比值以评估抗凋亡活性[2] - 人少突胶质前体细胞(OPC)活力检测:人OPCs在添加生长因子(EGF和FGF-2)的OPC生长培养基中培养,以2×104细胞/cm2的密度接种于96孔板。24小时后,OPCs分别用仅α-突触核蛋白原纤维(500 nM)或α-突触核蛋白原纤维联合雷沙吉兰(Rasagiline) (2 μM、5 μM)处理72小时。采用MTT法检测细胞活力:每孔加入10 μL MTT溶液(5 mg/mL),37°C孵育4小时后,用100 μL DMSO溶解甲瓒结晶,在酶标仪上测定570 nm处吸光度。活力以未处理对照组的百分比表示[3] |
| 动物实验 |
Animal/Disease Models: (PLP)-α-synuclein transgenic mice over 6 months of age[3]
Doses: Low-(0.8 mg/kg bw) and high dose (2.5 mg/kg bw) Route of Administration: Administered subcutaneously (sc) every 24 h for a total period of 4 weeks (from day 1 till day 28 of the experiment). Experimental Results: Low dose treatment did not show protective efficacy in striatum with number of neurons similar to placebo treated MSA mice. High dose was associated with about 15% rescue of DARPP-32 immunoreactive striatal neurons. Low dose treatment had no effect on nigral neuronal loss, but high dose completely protected nigral neurons with numbers comparable to healthy controls. Determination of inhibition of MAO activity in vivo [1] In in vivo studies, drugs were administered orally by gavage (p.o.). The animals weighed 250 – 300 g at the time of killing. For estimation of in vivo inhibitory effect, varying doses of the inhibitors were administered to groups of five or six rats for the stated times, the animals were killed by decapitation, tissues removed and frozen at −20°C, and enzyme activity determined subsequently as above. Enzyme activity in drug-treated tissues were expressed as a percentage of that in control tissues. Transgenic MSA mouse model experiment: Transgenic mice overexpressing human α-synuclein (line M83, 8-10 weeks old, male) were randomly divided into three groups: untreated transgenic group, Rasagiline-treated transgenic group, and wild-type control group (n=12 per group). Rasagiline was dissolved in 0.9% normal saline and administered orally via gavage at a dose of 1 mg/kg/day for 12 weeks. The untreated transgenic group and wild-type control group received the same volume of normal saline by gavage. During the treatment period, motor function was evaluated weekly using the rotarod test (speed increased from 4 to 40 rpm over 5 minutes, latency to fall was recorded) and the beam-walking test (number of hindlimb slips while traversing a 100 cm-long beam with 5 mm width was counted). After 12 weeks, mice were euthanized, and brains were harvested. Brain tissues were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned (5 μm thickness). Immunohistochemical staining was performed using antibodies against tyrosine hydroxylase (TH, a marker of dopaminergic neurons) and α-synuclein to quantify dopaminergic neuron loss in the SNpc and α-synuclein inclusions in oligodendrocytes [3] - Dexamethasone-induced brain apoptosis rat model experiment: Sprague-Dawley rats (6-8 weeks old, male) were randomly divided into four groups: normal control group, dexamethasone-only group, Rasagiline + dexamethasone group, and selegiline + dexamethasone group (n=10 per group). Dexamethasone was dissolved in 0.9% normal saline and administered subcutaneously at a dose of 0.5 mg/kg/day for 7 days. Rasagiline was dissolved in normal saline and administered orally via gavage at doses of 0.5 mg/kg/day and 1 mg/kg/day for 7 days (starting 1 day before dexamethasone treatment). Selegiline was administered orally at 1 mg/kg/day for 7 days (same schedule as Rasagiline). The normal control group received subcutaneous and oral normal saline. After treatment, rats were euthanized, and brains were removed. Hippocampal tissues were dissected, fixed with 4% paraformaldehyde, and processed for paraffin sectioning. TUNEL staining was performed to detect apoptotic neurons in the hippocampal CA1 region, and the number of TUNEL-positive cells was counted under a light microscope (five sections per rat) [2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rasagiline is rapidly absorbed following oral administration. The absolute bioavailability of rasagiline is about 36%. Rasagiline undergoes almost complete biotransformation in the liver prior to excretion. Glucuronide conjugation of rasagiline and its metabolites, with subsequent urinary excretion, is the major elimination pathway. After oral administration of 14C-labeled rasagiline, elimination occurred primarily via urine and secondarily via feces (62% of total dose in urine and 7% of total dose in feces over 7 days), with a total calculated recovery of 84% of the dose over a period of 38 days. Less than 1% of rasagiline was excreted as unchanged drug in urine. 87 L After oral administration of (14)C-labeled rasagiline, elimination occurred primarily via urine and secondarily via feces (62% of total dose in urine and 7% of total dose in feces over 7 days), with a total calculated recovery of 84% of the dose over a period of 38 days. Less than 1% of rasagiline was excreted as unchanged drug in urine. Rasagiline is rapidly absorbed; following oral administration, peak plasma concentrations are achieved in approximately 1 hour. The absolute bioavailability of rasagiline is about 36%. Following administration with a high-fat meal, peak plasma rasagiline concentrations and area under the plasma concentration-time curve (AUC) decreased by approximately 60 and 20%, respectively; because AUC is not substantially affected, rasagiline may be administered with or without food. Rasagiline readily crosses the blood-brain barrier. The mean steady-state or terminal half-life of rasagiline is 31 or 1.342 hours, respectively; however, there is no correlation between rasagiline's pharmacokinetic profile and its pharmacologic effects because the drug irreversibly inhibits MAO-B, and restoration of normal enzyme activity depends on the rate of de novo enzyme synthesis. Rasagiline is approximately 88-94% bound to plasma proteins, with 61-63% bound to albumin. IV studies in rats and dogs show that the volume of distribution (Vd) of rasagiline is several times that of total body water, indicating extensive tissue distribution. Tissue distribution of (14)C-rasagiline was studied in albino and pigmented rats, revealing peaks of tissue radioactivity between 0.25 and 0.5 hours. Distribution to large intestine, urinary bladder and lacrimal glands takes longer, whilst persistence (up to 24 hrs) was seen in eyes, skin and arterial walls of pigmented animals. In-vitro protein binding in plasma of animals is in the range of 70 to 90% and in human plasma in the range of 88 to 94%. Oral studies with (14)C-rasagiline show that absorption is rapid in all species, with Cmax attained in less than 2 hours. Absolute bioavailability has been estimated as 53-69% in rats, 13-22% in dogs, and 36% in humans. Toxicokinetic analyses during the toxicology studies showed that exposure was linear at doses higher than the pharmacological selectivity for inhibition of MOA-B and was maintained up to about 5 mg/kg/day. However, kinetics became non-linear at higher doses, possibly indicating saturation of the elimination processes for both rasagiline and its metabolite aminoindan. Accumulation was seen only at the highest doses in the mouse and dog studies (60 and 21 mg/kg/day respectively). For more Absorption, Distribution and Excretion (Complete) data for RASAGILINE (6 total), please visit the HSDB record page. Metabolism / Metabolites Rasagiline undergoes almost complete biotransformation in the liver prior to excretion. In vitro experiments indicate that both routes of rasagiline metabolism are dependent on the cytochrome P450 (CYP) system, with CYP 1A2 being the major isoenzyme involved in rasagiline metabolism. Rasagiline is extensively metabolized in the liver following oral administration. In vitro studies have shown that CYP1A2 is the predominant P450 isoform involved in the metabolic elimination of rasagiline. The primary human plasma metabolite formed following biotransformation of rasagiline is aminoindan. The proposed principal biotransformation pathways of rasagiline in human are N-dealkylation, hydroxylation of the indan ring, along with Phase II N or O-conjugation, including N-glucuronidation of the parent drug and of its metabolites. There was no bioconversion of rasagiline mesylate (R enantiomer) to its S enantiomer within the human body, as determined in plasma samples for healthy volunteers dosed with rasagiline. Rasagiline is not metabolized to amphetamine or methamphetamine. An extensive first pass metabolism effect is evident, likely due to rasagiline binding to MAO sites in the intestine prior to passing the liver. Metabolism is rapid and extensive, with a similar profile in all tested species. The primary route of biotransformation is via N-dealkylation to form aminoindan and by hydroxylation to form 3-hydroxy-N-propargyl-1-aminoindan. Conjugation by sulfide or glucuronic acid occurs. Microsomal studies indicate CYP1A2 as the primary metabolising isotype, but rasagiline is neither an inducer nor inhibitor of cytochrome p450. The metabolism of rasagiline under inhibition, induction of CYP1A2 or in presence of concomitant substrate to the enzyme has been addressed clinically. Rasagiline undergoes almost complete biotransformation in the liver prior to excretion. The metabolism of rasagiline proceeds through two main pathways: N-dealkylation and/or hydroxylation to yield 1-aminoindan (AI), 3-hydroxy-N-propargyl-1 aminoindan (3-OH-PAI) and 3-hydroxy-1-aminoindan (3-OH-AI). In vitro experiments indicate that both routes of rasagiline metabolism are dependent on the cytochrome P450 (CYP) system, with CYP 1A2 being the major isoenzyme involved in rasagiline metabolism. Glucuronide conjugation of rasagiline and its metabolites, with subsequent urinary excretion, is the major elimination pathway. Biological Half-Life Rasagiline has a mean steady-state half life of 3 hours but there is no correlation of pharmacokinetics with its pharmacological effect because of its irreversible inhibition of MAO-B. Rasagiline's mean steady-state half life is 3 hours ... . Rasagiline is eliminated with a half life of about 0.6 - 2 hours and ranging from 0.3 to 3.5 hours across the 0.5 to 20 mg dose range examined following oral administration. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Rasagiline has been reported to cause serum enzyme elevations in a small proportion of patients treated long term, although the abnormalities were usually mild and self-limiting. Rasagiline has not been implicated in cases of acute liver injury, but such instances have been reported with other less specific MAO inhibitors. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No clinical use of rasagiline during breastfeeding has been reported. Rasagiline might reduce serum prolactin and interfere with milk production. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Animal studies show that rasagiline reduces serum prolactin. The clinical relevance of these findings in nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding Plasma protein binding ranges from 88-94% with mean extent of binding of 61-63% to human albumin over the concentration range of 1-100 ng/ml. Interactions Antidepressant Agents, Selective Serotonin-reuptake Inhibitors (SSRIs): Potential pharmacologic interaction resembling serotonin syndrome (hyperthermia, rigidity, myoclonus, autonomic instability with rapid vital sign fluctuations, and mental status changes that may progress to extreme agitation, delirium, coma, and death). Concomitant use generally should be avoided. At least 14 days should elapse between discontinuance of rasagiline and initiation of an SSRI.Because both fluoxetine and its principal metabolite have relatively long half-lives, the manufacturer of rasagiline recommends that at least 5 weeks (or longer with high-dose or long-term fluoxetine therapy) elapse between discontinuance of fluoxetine therapy and initiation of rasagiline. Inhibitors of CYP1A2: Pharmacokinetic interaction observed during concomitant use with ciprofloxacin (increased plasma rasagiline concentrations). Dosage of rasagiline should be limited ... in patients receiving the drug concomitantly with ciprofloxacin or other CYP1A2 inhibitors. St. John's wort (Hypericum perforatum): Concomitant use is contraindicated. Potential pharmacologic interaction (resembling serotonin syndrome) with meperidine (coma, severe hypertension or hypotension, severe respiratory depression, seizures, malignant hyperpyrexia, excitation, peripheral vascular collapse, and death). Concomitant use with meperidine, methadone, propoxyphene, or tramadol is contraindicated. At least 14 days should elapse between discontinuance of rasagiline and initiation of meperidine. For more Interactions (Complete) data for RASAGILINE (12 total), please visit the HSDB record page. In the in vitro studies, Rasagiline at concentrations up to 10 μM did not induce cytotoxicity in primary rat cortical neurons or human OPCs (viability >90% compared to the untreated control group) [2][3] - In the in vivo studies, Rasagiline administered at doses of 0.5 mg/kg/day to 1 mg/kg/day (oral) for 7-12 weeks did not cause significant changes in body weight, food intake, or serum levels of liver enzymes (ALT, AST) and kidney function markers (BUN, creatinine) in rats or transgenic mice. No obvious histopathological damage was observed in the liver, kidney, or brain tissues of Rasagiline-treated animals [2][3] |
| 参考文献 |
|
| 其他信息 |
Drug Indication
Azilect is indicated for the treatment of idiopathic Parkinson's disease (PD) as monotherapy (without levodopa) or as adjunct therapy (with levodopa) in patients with end-of-dose fluctuations. The present study has demonstrated that rasagiline, like selegiline, is an irreversible inhibitor of MAO-B. This was demonstrated in experiments where rasagiline MAO inhibitory activity was examined in vitro and in vivo when it was given p.o. and MAO-A and -B were then estimated ex vivo in various tissues, at time intervals up to 13 days after rasagiline treatment. It is apparent that rasagiline is a very potent selective MAO-B inhibitor and has a good uptake across the blood-brain barrier, as shown by the similarity of inhibition curves between liver and brain. Although when compared in vitro, rasagiline had similar potency to selegiline for inhibition of MAO-B, the in vivo study showed a greater potency of rasagiline. This greater potency of rasagiline is even more marked if, instead of 50% enzyme inhibition, the dose required for 80% inhibition is measured. The reason for this is not currently known, but may be due to different rates of metabolism of the parent compounds in vivo, or to improved tissue penetration of rasagiline. Interestingly, preliminary studies in humans show an approximately 5 fold greater potency for rasagiline over selegiline for inhibition of platelet MAO-B (unpublished data). Although rasagiline has a greater potency than selegiline, its selectivity for MAO-A and -B inhibition is very similar to what has been reported for selegiline. However, in contrast to selegiline which does not show selectivity between its optical isomers for inhibition of MAO-A and -B, AGN 1135 shows roughly 4 and 2 orders of magnitude between its optical isomers for inhibition of MAO-B and -A respectively. The present results complement findings in non-human primate (monkey) brains (Gotz et al., 1998) where rasagiline was given chronically for 7 days at various doses and MAO-A and -B activities were measured in several brain regions, including caudate nucleus, globus pallidus, cerebral cortex and hippocampus. Rasagiline was shown to be a potent selective inhibitor of MAO-B in the caudate nucleus and globus pallidus where the activity of MAO-B is 4 fold higher than that of MAO-A (Gotz et al., 1998). The recovery of the MAO-A and -B activities after in vivo inhibition, which is related to the synthesis of enzyme apoprotein, differs between the tissues (liver, intestine and brain) examined. The small intestine MAO-B activity has the fastest recovery, while the brain MAO-B activity shows the slowest recovery. These differences in rat tissue enzyme activity recovery after rasagiline treatment, are not unusual since similar findings have been reported for enzyme recovery after inhibition by selegiline and clorgyline (Neff & Goridis, 1972; Della Corte & Tipton, 1980). Indeed, in primate (monkeys and human) brains the half-life for recovery of MAO-B after selegiline treatment has been reported to be well over 30 days (Fowler et al., 1994), and for the rat brain 13 days (Neff & Goridis, 1972; Della Corte & Tipton, 1980). In conclusion, the present study has shown that rasagiline is a potent irreversible inhibitor of MAO-B and it is 3 – 15 times more potent than selegiline in the rat in vivo with a similar selectivity for inhibition of MAO-B to MAO-A. Because of its cleaner pharacological profile, with absence of amphetamine-like properties, formation of the metabolite aminoindan rather than 1-methamphetamine, and recently described neuroprotective and antiapoptotic properties (Finberg et al., 1998; Huang et al., 1999; Youdim et al., 1999), we can conclude that this drug may have a preferential activity to that of selegiline in the treatment of Parkinson's disease.[1] We report here for the first time that rasagiline, selegiline, and 1-R-aminoindan significantly prevent dexamethasone-induced brain cell death involving in both neuroblastoma and glioblastoma cells. Among the three compounds, rasagiline has the highest neuroprotective effect compared to either selegiline or 1-R-aminoindan. Rasagiline (Azilect) and selegiline (1-deprenyl or Emsam) are irreversible inhibitors of MAO B. The greater neuroprotective quality of rasagiline may in part be due to the effects of the parent compound and its major metabolite, 1-R-aminoindan. Furthermore, the inhibitory effects of these drugs on MAO B catalytic activity and on apoptotic DNA fragment damage (observed by TUNEL staining) were examined. Rasagiline has shown the highest inhibition on MAO B enzymatic activity (Youdim et al. 2001a) and also has shown the highest prevention on apoptosis compared to selegiline and 1-R-aminoindan. The mechanism by which rasagiline and selegiline initiate their anti-apoptotic effect can be summarized by their up regulation of anti-apoptotic Bcl-2 and Bcl-Xl and down regulation of propaoptotic Bad, Bax, PARP, and caspase 3 (see Youdim et al. 2005a) and Youdim et al. 2006) for reviews). Because Bcl-2 and caspase 3 are key factors for preventing or mediating the mitochondrial-involved apoptosis (Lakhani et al. 2006), it suggests that the MAO inhibitors may protect cells from apoptosis through a mechanism involving the maintenance of mitochondrial homeostasis (Malorni et al. 1998). In addition, structure activity studies with rasagiline have shown that it is propargylamine moiety that produces this effect, since propargylamine which has little or no MAO inhibitory activity has a similar mechanism of neuroprotective activity with similar potency (Bar-Am et al. 2005). Furthermore, both rasagiline and proppargylamine activate neuroprotective protein kinase C (PKCα and PKCε), while down-regulating propaoptotic PKCδ and γ. Inhibition of PKC by GF109203X prevents their neuroprotective activity (Weinreb et al. 2005; Youdim et al. 2005a). The mechanism by which aminoindan has been fully elucidated. The neuroprotective properties of 1-R-aminoindan have been assessed employing a cytotoxic model of human neuroblastoma SKN-SH cells in high-density culture-induced neuronal death and in response to 6-hydroxydopamine. We show that 1-R-aminoindan (0.1–1 µM) significantly reduced the apoptosis-associated phosphorylated protein, H2A.X (Ser139), decreased the cleavage of caspase 9 and caspase 3, while increasing the anti-apoptotic proteins, Bcl-2 and Bcl-xl. Protein kinase C (PKC) inhibitor, GF109203X, prevented the neuroprotection, indicating the involvement of PKC in aminoindan-induced cell survival. Aminoindan markedly elevated pPKC (pan) and specifically that of the pro-survival PKC isoform, PKC epsilon (Bar-Am et al. 2007). In summary, the neurorptoective activity seen with rasagiline and its major metabolite, 1-R-aminoindan in the present and previous studies, may have relevance to the recent prospective clinical study in Parkinsonian subjects, ADAGIO, where rasagiline indicated that early treatment with rasagiline provided benefits that were not obtained with later initiation of the drug. This is the first time that a prospective large-scale, randomized, double-blind trial has provided evidence that a drug might slow down PD progression via neuroprotection (Hughes 2008).[2] Rasagiline is a propargylamine derivative with a chemical structure of N-propargyl-1R(+)-aminoindan, and its selective inhibition of mitochondrial MAO-B is thought to contribute to its neuroprotective effects by reducing the production of reactive oxygen species (ROS) generated from MAO-B-mediated monoamine metabolism [1] - In the dexamethasone-induced brain apoptosis model, the neuroprotective effect of Rasagiline was observed to be independent of its MAO-B inhibitory activity, as its metabolite aminoindan (which has no MAO-B inhibitory activity) also exhibited partial neuroprotective effects, suggesting that Rasagiline may exert neuroprotection through multiple mechanisms (including anti-apoptotic and antioxidant pathways) [2] - In the transgenic MSA model, Rasagiline not only protected dopaminergic neurons but also improved oligodendrocyte function by reducing α-synuclein aggregation, indicating its potential therapeutic value for neurodegenerative diseases associated with α-synuclein pathology (such as MSA and Parkinson's disease) [3] |
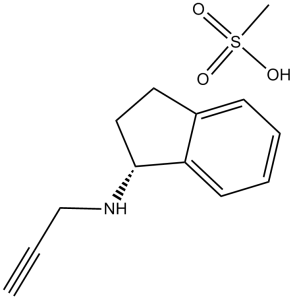
| 分子式 |
C12H13N.CH4O3S
|
|
|---|---|---|
| 分子量 |
267.34
|
|
| 精确质量 |
267.092
|
|
| 元素分析 |
C, 58.40; H, 6.41; N, 5.24; O, 17.95; S, 11.99
|
|
| CAS号 |
161735-79-1
|
|
| 相关CAS号 |
Rasagiline;136236-51-6;Rasagiline-13C3 mesylate;1391052-18-8;Rasagiline-13C3 mesylate racemic;1216757-55-9
|
|
| PubChem CID |
3052775
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.05 g/cm3
|
|
| 沸点 |
305.5ºC at 760 mmHg
|
|
| 熔点 |
155-158°C
|
|
| 闪点 |
146.8ºC
|
|
| 蒸汽压 |
0.000816mmHg at 25°C
|
|
| LogP |
2.872
|
|
| tPSA |
74.78
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
18
|
|
| 分子复杂度/Complexity |
305
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
CS(=O)(=O)O.C#CCN[C@@H]1CCC2=CC=CC=C12
|
|
| InChi Key |
JDBJJCWRXSVHOQ-UTONKHPSSA-N
|
|
| InChi Code |
InChI=1S/C12H13N.CH4O3S/c1-2-9-13-12-8-7-10-5-3-4-6-11(10)12;1-5(2,3)4/h1,3-6,12-13H,7-9H2;1H3,(H,2,3,4)/t12-;/m1./s1
|
|
| 化学名 |
methanesulfonic acid;(1R)-N-prop-2-ynyl-2,3-dihydro-1H-inden-1-amine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (374.06 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: Saline: 30 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7406 mL | 18.7028 mL | 37.4056 mL | |
| 5 mM | 0.7481 mL | 3.7406 mL | 7.4811 mL | |
| 10 mM | 0.3741 mL | 1.8703 mL | 3.7406 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03727139 | Completed Has Results | Drug: Rasagiline | Parkinson's Disease | Takeda | November 1, 2018 | |
| NCT01879748 | Completed | Drug: Rasagiline Drug: Placebo |
Parkinson's Disease | Teva Branded Pharmaceutical Products R&D, Inc. |
June 2013 | Phase 1 |
| NCT01032486 | Completed | Drug: Rasagiline mesylate | Parkinson's Disease | Teva Branded Pharmaceutical Products R&D, Inc. |
December 2009 | |
| NCT00203164 | Completed | Drug: rasagiline mesylate | Parkinson's Disease | Teva Branded Pharmaceutical Products R&D, Inc. |
May 2002 | Phase 3 |