| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
| 靶点 |
- Muscarinic acetylcholine receptors [1]
- 5-HT3 receptors (pKi = 6.3) [1] |
|---|---|
| 体外研究 (In Vitro) |
拮抗5-HT3受体:东莨菪碱(Scopolamine)作为5-HT3受体的竞争性拮抗剂发挥作用。在使用[3H]格拉司琼的放射性配体结合实验中,它以浓度依赖的方式抑制与5-HT3受体的结合。在表达人5-HT3A受体的细胞功能实验中,它拮抗5-HT诱导的内向电流,且这种拮抗作用是可克服的,表明存在竞争性相互作用 [1]
在表达 5-HT3 阻断的卵母细胞中,单独使用东莨菪碱不会引起反应;然而,当东莨菪碱与2μM 5-HT同时使用时,根据浓度不同,反应会受到抑制。当n = 6时,东莨菪碱pIC50值为5.68±0.05 (IC50=2.09) μM,Hill Slope为1.06±0.05,Kb为3.23 μM。在 5-HT 给药期间应用东莨菪碱,观察到相同的浓度依赖性效应。为了对与5-HT3受体的竞争性结合进行额外的测试,使用众所周知的高亲和力竞争性拮抗剂[3H]格拉司琼来评估未标记的东莨菪碱的竞争性。东莨菪碱的平均 pKi 为 5.17±0.24(Ki=6.76 μM,n=3),在 0.6 nM [3H]格拉司琼 (~Kd) 下表现出浓度营养竞争[1]。 |
| 体内研究 (In Vivo) |
在阿尔茨海默病的动物模型中,东莨菪碱可用于创建该疾病的模型。在组织病理学研究中,大脑的组织学没有显着变化;然而,仅接受自来水的霉菌的海马细胞中乙酰胆碱酯酶(AchE)活性(7.98±0.065;P<0.001)与正常组(3.06±0.296)相比。此外,与正常组(12.82±2.86)相比,东莨菪碱治疗组动物的丙二醛(MDA)水平显着升高(34.61±4.85;P<0.01)。东莨菪碱治疗组(0.3906±0.02)仍显示谷胱甘肽(GSH)原型;与正常组(43.21±3.46)相比,镜下淀粉样蛋白(Aβ1-42)浓度.
- 诱导小鼠健忘症:给小鼠注射东莨菪碱(Scopolamine)(1 mg/kg,腹腔注射)可诱导健忘症,表现为在Y迷宫测试中自发交替行为减少,在高架十字迷宫测试中转移潜伏期延长。这种健忘效应与胆碱能功能障碍相关 [2] - 损害大鼠记忆:给大鼠注射东莨菪碱(Scopolamine)(1 mg/kg,腹腔注射)会损害记忆,在Morris水迷宫测试中表现为逃避潜伏期延长、在目标象限停留时间减少,在高架十字迷宫测试中转移潜伏期延长。这种记忆损害与脑内乙酰胆碱水平降低有关 [3] - 酶学实验 - 5-HT3受体放射性配体结合实验:将表达5-HT3受体的细胞 membranes与[3H]格拉司琼在不同浓度的东莨菪碱(Scopolamine)存在下共同孵育。孵育后,过滤混合物以分离结合态和游离态配体,测量结合态配体的放射性,据此计算抑制常数(pKi) [1] - 5-HT3受体拮抗功能实验:采用膜片钳技术对表达人5-HT3A受体的细胞进行电压钳制。施加5-HT诱导内向电流,通过将东莨菪碱(Scopolamine)与不同浓度的5-HT共同施加,评估其对这些电流的影响。分析浓度-反应曲线以确定拮抗性质 [1] |
| 动物实验 |
- Amnesia induction in mice: Mice are divided into groups, with one group receiving Scopolamine (1 mg/kg, i.p.) 30 minutes before behavioral tests. The Y-maze test is conducted to assess spontaneous alternation behavior, where mice are allowed to explore the maze for 5 minutes, and the alternation percentage is calculated. The elevated plus maze test is performed by placing mice on the open arm and measuring the transfer latency to move to the closed arm [2]
- Memory impairment in rats: Rats are administered Scopolamine (1 mg/kg, i.p.) 30 minutes prior to behavioral tests. The Morris water maze test is used, where rats are trained to find a hidden platform, and escape latency is recorded. On the probe day, the platform is removed, and the time spent in the target quadrant is measured. The elevated plus maze test is conducted by placing rats on the open arm, and transfer latency is recorded [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The pharmacokinetics of scopolamine differ substantially between different dosage routes. Oral administration of 0.5 mg scopolamine in healthy volunteers produced a Cmax of 0.54 ± 0.1 ng/mL, a tmax of 23.5 ± 8.2 min, and an AUC of 50.8 ± 1.76 ng\*min/mL; the absolute bioavailability is low at 13 ± 1%, presumably because of first-pass metabolism. By comparison, IV infusion of 0.5 mg scopolamine over 15 minutes resulted in a Cmax of 5.00 ± 0.43 ng/mL, a tmax of 5.0 min, and an AUC of 369.4 ± 2.2 ng\*min/mL. Other dose forms have also been tested. Subcutaneous administration of 0.4 mg scopolamine resulted in a Cmax of 3.27 ng/mL, a tmax of 14.6 min, and an AUC of 158.2 ng\*min/mL. Intramuscular administration of 0.5 scopolamine resulted in a Cmax of 0.96 ± 0.17 ng/mL, a tmax of 18.5 ± 4.7 min, and an AUC of 81.3 ± 11.2 ng\*min/mL. Absorption following intranasal administration was found to be rapid, whereby 0.4 mg of scopolamine resulted in a Cmax of 1.68 ± 0.23 ng/mL, a tmax of 2.2 ± 3 min, and an AUC of 167 ± 20 ng\*min/mL; intranasal scopolamine also had a higher bioavailability than that of oral scopolamine at 83 ± 10%. Due to dose-dependent adverse effects, the transdermal patch was developed to obtain therapeutic plasma concentrations over a longer period of time. Following patch application, scopolamine becomes detectable within four hours and reaches a peak concentration (tmax) within 24 hours. The average plasma concentration is 87 pg/mL, and the total levels of free and conjugated scopolamine reach 354 pg/mL. Following oral administration, approximately 2.6% of unchanged scopolamine is recovered in urine. Compared to this, using the transdermal patch system, less than 10% of the total dose, both as unchanged scopolamine and metabolites, is recovered in urine over 108 hours. Less than 5% of the total dose is recovered unchanged. The volume of distribution of scopolamine is not well characterized. IV infusion of 0.5 mg scopolamine over 15 minutes resulted in a volume of distribution of 141.3 ± 1.6 L. IV infusion of 0.5 mg scopolamine resulted in a clearance of 81.2 ± 1.55 L/h, while subcutaneous administration resulted in a lower clearance of 0.14-0.17 L/h. Scopolamine hydrobromide is rapidly absorbed following IM or subcutaneous injection. The drug is well absorbed from the GI tract, principally from the upper small intestine. Scopolamine also is well absorbed percutaneously. Following topical application behind the ear of a transdermal system, scopolamine is detected in plasma within 4 hours, with peak concentrations occurring within an average of 24 hours. In one study in healthy individuals, mean free and total (free plus conjugated) plasma scopolamine concentrations of 87 and 354 pg/mL, respectively, have been reported within 24 hours following topical application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours. /Scopolamine hydrobromide/ Following oral administration of a 0.906-mg dose of scopolamine in one individual, a peak concentration of about 2 ng/mL was reached within 1 hour. Although the commercially available transdermal system contains 1.5 mg of scopolamine, the membrane-controlled diffusion system is designed to deliver approximately 1 mg of the drug to systemic circulation at an approximately constant rate over a 72-hour period. An initial priming dose of 0.14 mg of scopolamine is released from the adhesive layer of the system at a controlled, asymptotically declining rate over 6 hours; then, the remainder of the dose is released at an approximate rate of 5 ug/hour for the remaining 66-hour functional lifetime of the system. The manufacturer states that the initial priming dose saturates binding sites on the skin and rapidly brings the plasma concentration to steady-state. In a crossover study comparing urinary excretion rates of scopolamine during multiple 12-hour collection intervals in healthy individuals, there was no difference between the rates of excretion of drug during steady-state (24-72 hours) for constant-rate IV infusion (3.7-6 mcg/hour) and transdermal administration. The transdermal system appeared to deliver the drug to systemic circulation at the same rate as the constant-rate IV infusion; however, relatively long collection intervals (12 hours) make it difficult to interpret the data precisely. During the 12- to 24-hour period of administration and after 72 hours, the rate of excretion of scopolamine was higher with the transdermal system than with the constant-rate IV infusion. The distribution of scopolamine has not been fully characterized. The drug appears to be reversibly bound to plasma proteins. Scopolamine apparently crosses the blood-brain barrier since the drug causes CNS effects. The drug also reportedly crosses the placenta and is distributed into milk.. Although the metabolic and excretory fate of scopolamine has not been fully determined, the drug is thought to be almost completely metabolized (principally by conjugation) in the liver and excreted in urine. Following oral administration of a single dose of scopolamine in one study, only small amounts of the dose (about 4-5%) were excreted unchanged in urine within 50 hours; urinary clearance of unchanged drug was about 120 mL/minute. In another study, 3.4% or less than 1% of a single dose was excreted unchanged in urine within 72 hours following subcutaneous injection or oral administration of the drug, respectively. Following application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours in healthy individuals, the urinary excretion rate of free and total (free plus conjugated) scopolamine was about 0.7 and 3.8 ug/hour, respectively. Following removal of the transdermal system of scopolamine, depletion of scopolamine bound to skin receptors at the site of the application of the transdermal system results in a log-linear decrease in plasma scopolamine concentrations. Less than 10% of the total dose is excreted in urine as unchanged drug and its metabolites over 108 hours. Metabolism / Metabolites Little is known about the metabolism of scopolamine in humans, although many metabolites have been detected in animal studies. In general, scopolamine is primarily metabolized in the liver, and the primary metabolites are various glucuronide and sulphide conjugates. Although the enzymes responsible for scopolamine metabolism are unknown, _in vitro_ studies have demonstrated oxidative demethylation linked to CYP3A subfamily activity, and scopolamine pharmacokinetics were significantly altered by coadministration with grapefruit juice, suggesting that CYP3A4 is responsible for at least some of the oxidative demethylation. Although the metabolic and excretory fate of scopolamine has not been fully determined, the drug is thought to be almost completely metabolized (principally by conjugation) in the liver and excreted in urine. Biological Half-Life The half-life of scopolamine differs depending on the route. Intravenous, oral, and intramuscular administration have similar half-lives of 68.7 ± 1.0, 63.7 ± 1.3, and 69.1 ±8/0 min, respectively. The half-life is greater with subcutaneous administration at 213 min. Following removal of the transdermal patch system, scopolamine plasma concentrations decrease in a log-linear fashion with a half-life of 9.5 hours. Following application of a single transdermal scopolamine system that delivered approximately 1 mg/72 hours, the average elimination half-life of the drug was 9.5 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of scopolamine during breastfeeding. Use during labor appears to have a detrimental effect on newborn infants' nursing behavior. Long-term use of scopolamine might reduce milk production or milk letdown, but a single systemic or ophthalmic dose is not likely to interfere with breastfeeding. During long-term use, observe for signs of decreased lactation (e.g., insatiety, poor weight gain). To substantially diminish the amount of drug that reaches the breastmilk after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Anticholinergics can inhibit lactation in animals, apparently by inhibiting growth hormone and oxytocin secretion. Anticholinergic drugs can also reduce serum prolactin in nonnursing women. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. A retrospective case-control study conducted in two hospitals in central Iran compared breastfeeding behaviors in the first 2 hours postdelivery by infants of 4 groups of primiparous women with healthy, full-term singleton births who had vaginal deliveries. The groups were those who received no medications during labor, those who received oxytocin plus scopolamine, those who received oxytocin plus meperidine, and those who received oxytocin, scopolamine and meperidine. The infants in the no medication group performed better than those in all other groups, and the oxytocin plus scopolamine group performed better than the groups that had received meperidine. Protein Binding Scopolamine may reversibly bind plasma proteins in humans. In rats, scopolamine exhibits relatively low plasma protein binding of 30 ± 10%. Interactions Scopolamine should be used with care in patients taking other drugs that are capable of causing CNS effects such as sedatives, tranquilizers, or alcohol. Special attention should be paid to potential interactions with drugs having anticholinergic properties; e.g., other belladonna alkaloids, antihistamines (including meclizine), tricyclic antidepressants, and muscle relaxants. The absorption of oral medications may be decreased during the concurrent use of scopolamine because of decreased gastric motility and delayed gastric emptying. Concomitant administration of antimuscarinics and corticosteroids may result in increased intraocular pressure. /Antimuscarinics/Antispasmodics/ Antacids may decrease the extent of absorption of some oral antimuscarinics when these drugs are administered simultaneously. Therefore, oral antimuscarinics should be administered at least 1 hour before antacids. Antimuscarinics may be administered before meals to prolong the effects of postprandial antacid therapy. However, controlled studies have failed to demonstrate a substantial difference in gastric pH when combined antimuscarinic and antacid therapy was compared with antacid therapy alone. /Antimuscarinics/Antispasmodics/ For more Interactions (Complete) data for SCOPOLAMINE (8 total), please visit the HSDB record page. Toxicity Summary Toxicity does not happen as frequently with the transdermal form of scopolamine due to its extended-release nature. Data on the toxic dose of scopolamine in the tablet form is scattered. Reports exist that 10 mg a day can be lethal for children. In adults, consumption of more than 100 mg a day did not result in death. The other feared toxidrome of scopolamine overdose is an anticholinergic syndrome resulting in tachycardia, hallucinations, hyperthermia, and dry membranes. Physostigmine 1 to 4 mg IV can serve as an antidote in such severe cases. However, with the transdermal application, only minor side effects are most commonly observed. Adverse Effects The most commonly reported side effects of scopolamine patch use are blurred vision, dilated pupils, and dry mouth. The vision disturbances are most often due to inadequate handwashing techniques after the application of the patch. Less frequently reported side effects are related to anticholinergic toxidrome: flushed skin, tachycardia, agitation, and confusion. These side effects are usually mild and quick to resolve after patch removal. If needed, the clinician can administer a reversal agent like physostigmine if a side effect persists. Interactions Scopolamine should be used with care in patients taking other drugs that are capable of causing CNS effects such as sedatives, tranquilizers, or alcohol. Special attention should be paid to potential interactions with drugs having anticholinergic properties; e.g., other belladonna alkaloids, antihistamines (including meclizine), tricyclic antidepressants, and muscle relaxants. The absorption of oral medications may be decreased during the concurrent use of scopolamine because of decreased gastric motility and delayed gastric emptying. Concomitant administration of antimuscarinics and corticosteroids may result in increased intraocular pressure. /Antimuscarinics/Antispasmodics/ Antacids may decrease the extent of absorption of some oral antimuscarinics when these drugs are administered simultaneously. Therefore, oral antimuscarinics should be administered at least 1 hour before antacids. Antimuscarinics may be administered before meals to prolong the effects of postprandial antacid therapy. However, controlled studies have failed to demonstrate a substantial difference in gastric pH when combined antimuscarinic and antacid therapy was compared with antacid therapy alone. /Antimuscarinics/Antispasmodics/ Antidote and Emergency Treatment Emergency and supportive measures: Maintain an open airway and assist ventilation if needed. Treat hyperthermia, coma, rhabdomyolysis, and seizures if they occur. /Anticholinergics/ Specific drugs and antidotes: A small dose of physostigmine .... can be given to patients with severe toxicity (e.g., hyperthermia, severe delirium, or tachycardia). Caution: Physostigmine can cause AV block, asystole, and seizures, especially in patients with tricyclic antidepressant overdose. Neostigmine, a peripherally acting cholinesterase inhibitor, may be useful in treating anticholinergic-induced ileus. /Anticholinergics/ Decontamination: Administer activated charcoal orally if conditions are appropriate. Gastric lavage is not necessary after small to moderate ingestions if activated charcoal can be given promptly. Because of slowed gastrointestinal motility, gut decontamination procedures may be helpful even in late-presenting patients. /Anticholinergics/ Enhanced elimination: Hemodialysis, hemoperfusion, peritoneal dialysis, and repeat-dose charcoal are not effective in removing anticholinergic agents. /Anticholinergics/ Human Toxicity Excerpts /HUMAN EXPOSURE STUDIES/ Scopolamine-induced deficits in cognitive and motor processes have been widely demonstrated in animals and humans, although the role of acetylcholine in working memory is not as well understood. This study examined the role of acetylcholine neurotransmission in visuospatial short term and working memory using the Groton Maze Learning Test (GMLT). The GMLT is a computerized hidden maze learning test that yields measures of component cognitive processes such as spatial memory, working memory, and visuomotor function, as well as their integration in trial-and-error problem solving. Healthy older adults were administered scopolamine (0.3 mg subcutaneous), the acetlycholinesterase inhibitor donepezil (5 mg oral), scopolamine with donepezil, or placebo. Compared to placebo, low-dose scopolamine led to performance deficits on all measures of the GMLT. The greatest scopolamine-induced deficits were observed in errors reflecting working memory processes (e.g., perseverative errors d=-2.98, and rule-break errors d=-2.49) and these impairments remained robust when statistical models accounted for scopolamine-related slowing in visuomotor speed. Co-administration of donepezil partially ameliorated scopolamine-related impairments and this effect was greatest for measures of working memory than short-term memory. By itself, donepezil was associated with a small improvement in visuomotor function. These results suggest that scopolamine disrupts processes required for rule maintenance and performance monitoring, in combination with visuomotor slowing and sequential location learning. PMID:18514746 /SIGNS AND SYMPTOMS/ High doses of scopolamine produce CNS effects (e.g., restlessness, disorientation, irritability, hallucinations) similar to those produced by toxic doses of other antimuscarinics. /SIGNS AND SYMPTOMS/ Scopolamine toxicity usually arises from adulterated products or ingestion of scopolamine-containing plants, producing classic anticholinergic syndrome. Adults as well as children have developed central anticholinergic syndrome with hallucinations and incontinence after being treated with a single transdermal patch. /SIGNS AND SYMPTOMS/ When transdermal scopolamine has been used for longer than 3 days, withdrawal of its use has occasionally been followed by dizziness, nausea, vomiting, headache, and disturbance of equilibrium. Non-Human Toxicity Excerpts /LABORATORY ANIMALS: Chronic Exposure or Carcinogenicity/ ... CONCLUSIONS: Under the conditions of these 2 year gavage studies, there was no evidence of carcinogenic activity of scopolamine hydrobromide trihydrate in male or female F344/N rats or B6C3F1 mice administered l, 5, or 25 mg/kg. /Scopolamine hydrobromide/ Toxicology & Carcinogenesis Studies of Scopolamine Hydrobromide in F344/N Rats and B6C3F1 Mice (Gavage Studies). Technical Report Series No. 445 (1997) NIH Publication No. 97-3361 U.S. Department of Health and Human Services, National /LABORATORY ANIMALS: Developmental or Reproductive Toxicity/ Reproductive studies in rats and rabbits using IV scopolamine hydrobromide at dosages producing plasma concentrations of the drug 100 times greater than those achievable after application of the transdermal system in humans have shown a marginal embryotoxic effect in rabbits; no teratogenic effects were observed in rats. /Scopolamine hydrobromide/ /LABORATORY ANIMALS: Developmental or Reproductive Toxicity/ Teratogenic studies were performed in pregnant rats and rabbits with scopolamine hydrobromide administered by daily intravenous injection. No adverse effects were recorded in rats. Scopolamine hydrobromide has been shown to have a marginal embryotoxic effect in rabbits when administered by daily intravenous injection at doses producing plasma levels approximately 100 times the level achieved in humans using a transdermal system. /Scopolamine hydrobromide/ /LABORATORY ANIMALS: Developmental or Reproductive Toxicity/ Fertility studies were performed in female rats and revealed no evidence of impaired fertility or harm to the fetus due to scopolamine hydrobromide administered by daily subcutaneous injection. Maternal body weights were reduced in the highest-dose group (plasma level approximately 500 times the level achieved in humans using a transdermal system). /Scopolamine hydrobromide/ Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 2192 |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Adjuvants, Anesthesia; Antiemetics; Muscarinic Antagonists; Mydriatics; Parasympatholytics Although transdermal scopolamine has been shown to decrease basal acid output and inhibit betazole-, pentagastrin-, and peptone-stimulated gastric acid secretion in healthy individuals, it has not been determined whether transdermal scopolamine is effective in the adjunctive treatment of peptic ulcer disease. /Use is not currently included in the labeling approved by the US FDA/ Transdermal scopolamine has shown minimal antiemetic activity against chemotherapy-induced vomiting. /Use is not currently included in the labeling approved by the US FDA/ Scopolamine hydrobromide is used as a mydriatic and cycloplegic, especially when the patient is sensitive to atropine or when less prolonged cycloplegia is required. The effects of the drug appear more rapidly and have a shorter duration of action than those of atropine. Scopolamine hydrobromide is also used in the management of acute inflammatory conditions (i.e., iridocyclitis) of the iris and uveal tract. /Scopolamine hydrobromide/ For more Therapeutic Uses (Complete) data for SCOPOLAMINE (10 total), please visit the HSDB record page. Drug Warnings The use of scopolamine to produce tranquilization and amnesia in a variety of circumstances, including labor, is declining and of questionable value. Given alone in the presence of pain or severe anxiety, scopolamine may induce outbursts of uncontrolled behavior. Scopolamine in therapeutic doses normally causes CNS depression manifested as drowsiness, amnesia, fatigue, and dreamless sleep, with a reduction in rapid eye movement (REM) sleep. It also causes euphoria and is therefore subject to some abuse. The depressant and amnesic effects formerly were sought when scopolamine was used as an adjunct to anesthetic agents or for preanesthetic medication. However, in the presence of severe pain, the same doses of scopolamine can occasionally cause excitement, restlessness, hallucinations, or delirium. These excitatory effects resemble those of toxic doses of atropine. Scopolamine-induced inhibition of salivation occurs within 30 minutes or within 30 minutes to 1 hour and peaks within 1 or 1-2 hours after IM or oral administration, respectively; inhibition of salivation persists for up to 4-6 hours. Following IV administration of a 0.6-mg dose in one study, amnesia occurred within 10 minutes, peaked between 50-80 minutes, and persisted for at least 120 minutes after administration. Following IM administration of a 0.2-mg dose of scopolamine in one study, antiemetic effect occurred within 15-30 minutes and persisted for about 4 hours. Following IM administration of a 0.1- or 0.2-mg dose in another study, mydriasis persisted for up to 8 hours. The transdermal system is designed to provide an antiemetic effect with an onset of about 4 hours and with a duration of up to 72 hours after application. Small doses of ... scopolamine inhibit the activity of sweat glands innervated by sympathetic cholinergic fibers, and the skin becomes hot and dry. Sweating may be depressed enough to raise the body temperature, but only notably so after large doses or at high environmental temperatures. For more Drug Warnings (Complete) data for SCOPOLAMINE (21 total), please visit the HSDB record page. Pharmacodynamics Scopolamine is an anticholinergic belladonna alkaloid that, through competitive inhibition of muscarinic receptors, affects parasympathetic nervous system function and acts on smooth muscles that respond to acetylcholine but lack cholinergic innervation. Formulated as a patch, scopolamine is released continuously over three days and remains detectable in urine over a period of 108 hours. Scopolamine is contraindicated in angle-closure glaucoma and should be used with caution in patients with open-angle glaucoma due to scopolamine's ability to increase intraocular pressure. Also, scopolamine exhibits several neuropsychiatric effects: exacerbated psychosis, seizures, seizure-like, and other psychiatric reactions, and cognitive impairment; scopolamine may impair the ability of patients to operate machinery or motor vehicles, play underwater sports, or perform any other potentially hazardous activity. Women with severe preeclampsia should avoid scopolamine. Patients with gastrointestinal or urinary disorders should be monitored frequently for impairments, and scopolamine should be discontinued if these develop. Scopolamine can cause blurred vision if applied directly to the eye, and the transdermal patch should be removed before an MRI procedure to avoid skin burns. Due to its gastrointestinal effects, scopolamine can interfere with gastric secretion testing and should be discontinued at least 10 days before performing the test. Finally, scopolamine may induce dependence and resulting withdrawal symptoms, such as nausea, dizziness, vomiting, gastrointestinal disturbances, sweating, headaches, bradycardia, hypotension, and various neuropsychiatric manifestations following treatment discontinuation; severe symptoms may require medical attention. Scopolamine is a muscarinic acetylcholine receptor antagonist that crosses the blood-brain barrier. It is commonly used in preclinical studies to induce memory impairment, mimicking the cholinergic dysfunction observed in Alzheimer's disease. Its ability to antagonize 5-HT3 receptors suggests potential interactions with serotonergic systems, which may contribute to its pharmacological effects. |
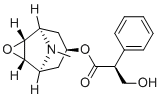
| 分子式 |
C17H21NO4
|
|---|---|
| 分子量 |
303.35
|
| 精确质量 |
303.147
|
| 元素分析 |
C, 67.31; H, 6.98; N, 4.62; O, 21.10
|
| CAS号 |
51-34-3
|
| 相关CAS号 |
Scopolamine hydrobromide;114-49-8;Scopolamine butylbromide;149-64-4;Scopolamine hydrobromide trihydrate;6533-68-2;Scopolamine hydrochloride;55-16-3
|
| PubChem CID |
11968014
|
| 外观&性状 |
White to off-white <55°C powder,>55°C liquid
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
460.3±45.0 °C at 760 mmHg
|
| 熔点 |
59ºC
|
| 闪点 |
232.2±28.7 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.614
|
| LogP |
0.76
|
| tPSA |
62.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
22
|
| 分子复杂度/Complexity |
418
|
| 定义原子立体中心数目 |
5
|
| SMILES |
O1[C@]2([H])[C@@]3([H])C([H])([H])C([H])(C([H])([H])[C@@]([H])([C@]12[H])N3C([H])([H])[H])OC([C@@]([H])(C1C([H])=C([H])C([H])=C([H])C=1[H])C([H])([H])O[H])=O
|
| InChi Key |
STECJAGHUSJQJN-SFSMXDMGSA-N
|
| InChi Code |
InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11?,12-,13-,14+,15+,16+/m1/s1
|
| 化学名 |
[(1R,2S,4S,5S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl] (2S)-3-hydroxy-2-phenylpropanoate
|
| 别名 |
Scopolamine; [(1R,2S,4S,5S)-9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl] (2S)-3-hydroxy-2-phenylpropanoate; 51-34-3; [(1S,2R,4S,5S)-9-Methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl] (2S)-3-hydroxy-2-phenylpropanoate; CHEMBL1187846; Transderm SCOP; l-Scopolamine; SEE
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~329.65 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.24 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.24 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (8.24 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2965 mL | 16.4826 mL | 32.9652 mL | |
| 5 mM | 0.6593 mL | 3.2965 mL | 6.5930 mL | |
| 10 mM | 0.3297 mL | 1.6483 mL | 3.2965 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04314713 | TERMINATED | Drug: Scopolamine Hydrobromide Trihydrate | Scopolamine Causing Adverse Effects in Therapeutic Use | Battelle Memorial Institute | 2020-06-02 | Phase 1 |
| NCT03029650 | COMPLETEDWITH RESULTS | Drug: Transderm Scop® Drug: Intravenous scopolamine hydrobromide |
Healthy | University of Iowa | 2016-11 | Phase 4 |
| NCT03874130 | UNKNOWN STATUS | Drug: Scopolamine | Major Depressive Disorder (MDD) | Repurposed Therapeutics, Inc. | 2018-08-01 | Phase 1 |
| NCT02516098 | COMPLETEDWITH RESULTS | Drug: hyoscine butylbromide | Healthy | Boehringer Ingelheim | 2015-10 | Phase 1 |
| NCT04349722 | COMPLETED | Drug: Hyoscine Butylbromide Other: Placebo |
Labor Long | National University of Malaysia | 2019-12-01 | Phase 4 |
|
|
|