| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |
| 靶点 |
Natural product; TMEM16A chloride channel; PKM2; NF-κB
|
|---|---|
| 体外研究 (In Vitro) |
Shikonin,TMEM16A 氯通道抑制剂,IC50 为 6.5 μM[1]。此外,Shikonin激肽特异性抑制 PKM2 [2]。此外,它还可以阻止核因子-κB (NF-κB) 通路的激活并抑制肿瘤坏死因子-α (TNF-α)。与对照相比,当暴露于浓度大于 50 μM 的紫草素时,正常人角质形成细胞 (NHK) 的活力显着降低 (P<0.05)。紫草素预处理两小时可抑制 TNF-α 诱导的 NF-κB p65 核转位 [3]。用 5 和 7.5 μM 紫草素处理 12 小时后,细胞活力显着降低。与 0 小时组相比,两种细胞系的抑制作用也显示出时间依赖性模式。结果发现,在24至48小时的时间段内,5μM紫草素比2.5μM紫草素具有更强的抑制作用。当U87和U251细胞用2.5、5和7.5 μM紫草素处理24和48小时(p<0.01)时,它们的侵袭性远低于对照组[4]。
在这项研究中,我们发现10μmol/L的Shikonin/紫草素刺激了正常人角质形成细胞的生长,1μmol/L的苦参素促进了人皮肤成纤维细胞的生长。然而,紫草素在体外不能直接诱导皮肤成纤维细胞中COL1 mRNA的表达和PIP的产生。此外,1μmol/L的紫草素抑制了肿瘤坏死因子-α刺激皮肤成纤维细胞诱导的NF-κB p65从细胞质向细胞核的易位。此外,紫草素抑制蛋白酶体的胰凝乳蛋白酶样活性,并与皮肤成纤维细胞中磷酸化抑制剂κB-α的积累有关。 结论:这些结果表明,Shikonin/紫草素可能通过其促进细胞生长的活性促进伤口愈合,并通过抑制蛋白酶体的活性抑制皮肤炎症。因此,紫草素可能是伤口愈合和炎症性皮肤病的潜在治疗试剂。[3] 紫草素/Shikonin是从紫草根中提取的蒽醌衍生物。紫草素传统上用于治疗炎症和感染性疾病,如肝炎。紫草素还抑制各种肿瘤的增殖并诱导其凋亡。然而,紫草素对胶质瘤的影响尚未完全阐明。本研究旨在探讨紫草素对人胶质母细胞瘤细胞迁移和侵袭的影响及其潜在机制。U87和U251人胶质母细胞瘤细胞用2.5、5和7.5μmol/L的紫草素处理,用CCK8、划痕愈合、体外Transwell迁移和侵袭试验评估细胞存活率、迁移和侵袭性。检测基质金属蛋白酶-2(MMP-2)和基质金属蛋白酶-9(MMP-9)的表达和活性,以及磷酸化β-catenin(p-β-catenmin)和磷酸化PI3K/Akt的表达。结果显示,紫草素显著抑制U87和U251细胞的增殖、迁移、侵袭和MMP-2和MMP-9的表达。β-catenin在两种细胞系中的表达趋势相反。它在U87细胞中受到显著抑制,在U251细胞中得到促进。本研究的结果表明,紫草素通过抑制MMP-2和-9的表达和活性,对胶质瘤细胞的迁移和侵袭具有抑制作用。此外,紫草素还抑制了p-PI3K和p-Akt的表达,以减弱两种细胞系中的细胞迁移和侵袭以及MMP-2和MMP-9的表达,这可以被PI3K/Akt通路激动剂胰岛素样生长因子-1(IGF-1)逆转。[4] 紫草素/Shikonin是一种高度亲脂性的萘醌,存在于紫草根中,在中药中具有多效性。基于其已报道的解热和抗炎特性,我们研究了紫草素是否抑制NLRP3炎性小体的激活。炎症小体是细胞质蛋白复合物,作为胱天蛋白酶-1募集和激活的支架,进而导致促炎细胞因子IL-1β和IL-18的切割和分泌。NLRP3炎性小体激活包括两个步骤:启动,即NF-κB通路的激活,以及炎性小体组装。虽然之前有报道称紫草素可以抑制启动步骤,但我们证明紫草素还可以抑制由可溶性和颗粒NLRP3激动剂在启动的永生化小鼠骨髓源性巨噬细胞中诱导的炎症小体激活的第二步。紫草宁比乙酰紫草宁更能有效地降低黑色素对NLRP3炎性体的激活。我们的研究结果表明,紫草素还可以通过双链DNA抑制AIM2炎性小体的激活。紫草素抑制小鼠巨噬细胞中ASC斑点的形成和caspase-1的激活,并抑制分离的caspase-1活性,表明它直接靶向caspase-1。将紫草素与β-乳球蛋白复合可以降低其毒性,同时保持对NLRP3炎性体激活的抑制作用,这表明具有提高生物利用度的紫草素可能对炎性体介导的疾病的治疗应用感兴趣[7]。 (-)-Alkannin 的 IC50 分别为 2.38 和 4.53 μM,抑制 HCT-116 和 SW-480 细胞的发育 [8]。 |
| 体内研究 (In Vivo) |
与骨关节炎组相比,紫草素显着阻止骨关节炎大鼠模型中IL-1β和TNF-α表达水平的升高(P<0.01)。在骨关节炎大鼠模型中,与骨关节炎组相比,紫草素显着降低了 NF-κB 蛋白表达量(P<0.01)。在用紫草素治疗的大鼠骨关节炎模型中,与骨关节炎组相比,诱导的iNOS水平降低(P<0.01)。与骨关节炎组相比,紫草素治疗显着降低了骨关节炎大鼠模型中COX-2蛋白表达的增加(P<0.01)。紫草素治疗的骨关节炎大鼠模型中caspase-3活性的增加明显低于骨关节炎组(P<0.01)。接受紫草素治疗后,骨关节炎组大鼠骨关节炎模型中Akt磷酸化显着恢复(P<0.01)[5]。
分泌性腹泻仍然是全球健康负担,并导致儿童死亡。抗腹泻疗法可能会减少腹泻疾病中的液体流失和肠道运动,这引起了人们的关注。在本研究中,我们使用基于细胞的荧光淬灭试验确定了紫草素是TMEM16A氯通道活性的抑制剂。紫草素的IC50值为6.5μM。短路电流测量表明,紫草素以剂量依赖的方式抑制Eact诱导的Cl(-)电流,IC50值为1.5μM。短路电流测量表明,紫草素对CCh诱导的小鼠结肠上皮细胞Cl(-)电流具有抑制作用,但不影响细胞质Ca(2+)浓度以及其他主要的肠细胞氯离子通道电导调节因子。特征研究发现,紫草素抑制基底外侧K(+)通道活性,而不影响Na(+)/K(+)-ATP酶活性。体内研究表明,紫草素显著延迟了小鼠的肠道运动,降低了轮状病毒腹泻新生小鼠模型的粪便含水量,而不影响体内病毒感染过程。综上所述,研究结果表明,紫草素抑制肠上皮细胞钙激活的氯通道,其抑制作用部分是通过抑制基底外侧K(+)通道活性实现的,紫草素可能是治疗轮状病毒分泌性腹泻的先导化合物。[1] 丙酮酸激酶M2(PKM2)的M2亚型已被证明在人类皮肤癌中上调。为了测试PKM2是否可能是化学预防的靶点,在一项化学诱导的小鼠皮肤癌变研究中使用了紫草素,紫草素是紫草根的天然产物,也是PKM2的特异性抑制剂。结果表明,紫草素治疗抑制了皮肤肿瘤的形成。皮肤表皮组织的形态学检查和免疫组织化学染色表明,紫草素抑制细胞增殖,但不诱导细胞凋亡。尽管紫草素单独抑制PKM2活性,但在皮肤癌变研究结束时,它并没有抑制肿瘤启动子诱导的皮肤表皮组织中PKM2的激活。为了揭示紫草素的潜在化学预防机制,进行了抗体微阵列分析,结果表明,化学致癌物处理上调了转录因子ATF2及其下游靶点Cdk4;而紫草素抑制了这些上调。在可促进的皮肤细胞模型中,ATF2的核水平在肿瘤促进过程中增加,而这种增加被紫草素抑制。此外,敲除ATF2降低了Cdk4和Fra-1(激活蛋白1的关键亚基)的表达水平。总之,这些结果表明,紫草素在皮肤癌变过程中抑制ATF2通路,而不是在体内抑制PKM2。[2] 紫草素先前已被证明具有抗肿瘤、抗炎、抗病毒和广泛的药理作用。本研究旨在探讨紫草素的保护作用是否通过抑制炎症和软骨细胞凋亡来介导,并阐明骨关节炎大鼠模型中的潜在分子机制。在健康雄性Sprague-Dawley大鼠中建立骨关节炎模型,每天腹腔注射10mg/kg紫草素4天。研究发现,紫草素治疗显著抑制了骨关节炎大鼠的炎症反应。与假手术组相比,骨关节炎显著增加了白细胞介素(IL)-1β、肿瘤坏死因子(TNF)-α和诱导型一氧化氮合酶(iNOS)水平。然而,紫草素治疗显著抑制了骨关节炎大鼠IL-1β、TNF-α和iNOS水平的升高。此外,与假手术组相比,骨关节炎大鼠的半胱氨酸天冬氨酸蛋白酶-3活性和环氧化酶(COX)-2蛋白表达显著增加,磷酸化Akt蛋白表达大大受到抑制。紫草素给药减轻了骨关节炎大鼠半胱氨酸天冬氨酸蛋白酶-3活性、COX-2表达和Akt磷酸化的变化。这些结果表明,紫草素通过调节骨关节炎大鼠模型中的磷酸肌醇3-激酶/Akt信号通路来抑制炎症和软骨细胞凋亡[5]。 |
| 酶活实验 |
碘化物流入荧光分析[1]
为了测量紫草素对TMEM16A的抑制作用,将表达TMEM16A的FRT细胞接种在黑壁透明底部96孔板中,直至融合。然后用PBS洗涤细胞三次,然后用不同浓度的紫草素孵育10分钟。用配备HQ 535/30M(535±15nm)发射和HQ500/20X(500±10nm)激发滤光片和注射泵的FLUOstar Galaxy微孔板读数器记录荧光数据。连续记录荧光14秒,以2秒的速度将ATP(200μM)与碘化物一起泵入系统。碘化物流入率(d[I-]/dt)如先前研究所述计算(Kristidis等人,1992)。 基于细胞的蛋白酶体活性测定[3] 在白壁96孔板中,约4000个HDF/孔在37°C下用0.1%DMSO、1μmol/L的Shikonin/紫草素或10μmol/L的内酰胺胱氨酸处理2小时,然后用50 ng/ml的TNF-α刺激30分钟。然后根据制造商的方案,将细胞与蛋白酶体Glo™细胞基检测试剂一起孵育15分钟。通过Centro LB 960微孔板光度计检测每个反应产生的发光。 Caspase-1活性测定[7] 使用BioVision的caspase-1抑制剂药物筛选试剂盒分析潜在的caspase-1抑制剂。紫草素和阳性抑制对照(Z-VAD-FMK)在PBS中制备,并应用于黑色96孔荧光板。加入活性胱天蛋白酶-1,然后加入胱天蛋白酶1底物YVAD-AFC。在37°C下孵育45分钟后,使用SinergyMx平板读数器测量样品的荧光。 |
| 细胞实验 |
免疫荧光研究[3]
将细胞接种到六孔板的盖玻片上,并在含有5%FBS的培养基中附着过夜。细胞在无血清培养基中饥饿24小时后,在用TNF-α(50 ng/ml)刺激之前,用1μmol/L的Shikonin/紫草素或0.1%的DMSO预处理细胞2小时。然后,在去除培养基后,用磷酸缓冲盐水冲洗细胞,并在4°C下用甲醇固定8分钟。在室温下用1%BSA的PBS溶液进行阻断步骤30分钟。细胞在室温下用1%BSA/PBS中的抗NF-κB p65(C-20)抗体(1:100稀释)免疫染色2小时,然后在室温下与FITC偶联的抗兔IgG-pAb(1:100稀释剂)孵育1小时。用BX 51TRF荧光显微镜观察载玻片。 免疫印迹分析[3] 人皮肤成纤维细胞用1μmol/L的Shikonin/紫草素或0.1%的DMSO预处理2小时,然后用50 ng/ml的TNF-α刺激30分钟。然后,根据制造商的说明,用核提取试剂盒提取细胞质蛋白。蛋白质在5-20%梯度凝胶上通过十二烷基硫酸钠聚丙烯酰胺凝胶电泳分离,并使用iBlot®系统通过半干转移法转移到硝化纤维膜上。 细胞增殖试验[4] 根据文献,用CCK8检测试剂盒测量细胞增殖。简而言之,将U87和U251细胞在标准DMEM中以每孔1×104个细胞的密度接种到96孔板中,并在标准条件下(37°C和5%CO2)孵育24小时。我们之前的数据显示,24小时后,Shikonin/紫草素的IC50值对U251细胞为1.84±0.34μmol/L,对U87细胞为2.02±0.44μmol/L。因此,本研究中使用的浓度为2.5、5和7.5μmol/L。然后将培养基替换为空白、无血清DMEM或含紫草素的DMEM,浓度分别为2.5、6和7.5μol/L。每个孔的总体积为200μL。将胶质瘤细胞在这些溶液中孵育0、12、24、36、48或72小时,然后在37°C下用每个孔中的20μL CCK8处理1.5小时。最后,轻轻摇动平板,使用ELISA平板读数器在570nm(OD570)处记录光密度。至少进行了三次独立实验。抑制率按以下公式计算:(ControlOD570-实验组OD570)/ControlOD570×100%。 体外迁移试验[4] 根据文献,在具有8-μm孔径Transwell插入物的24孔板中评估了人胶质母细胞瘤细胞的迁移能力。将亲本U87或U251细胞胰蛋白酶化,以5×105/mL的密度重新悬浮在无血清DMEM中,并将200μL细胞悬浮液加入上室。将500微升条件培养基(DMEM培养基,补充10%FBS)放入下腔室,作为细胞迁移的诱导剂。无血清DMEM作为阴性对照。将紫草素以2.5、5或7.5μmol/L的浓度添加到亲本U87细胞或U251细胞的悬浮液中。还添加了PIRES2-p-β-catenin、shRNA-p-β-cantin、LY294002(20μmol/L)或紫草素(5μmol/L)与PI3K/Akt激动剂胰岛素样生长因子-1(IGF-1)(20μg/mL,Proteintech)的组合。孵育24或48小时后,取出插入物,用棉签仔细去除过滤器上表面残留的细胞。迁移到下侧表面的细胞用PBS轻轻洗涤一次,在室温下用甲醇和冰醋酸(按3:1混合)固定30分钟,并在Giemsa染色中染色15分钟。在六个随机高倍视野(×400)中计数迁移细胞的平均数量。 划痕愈合试验[4] 如前所述,进行划痕愈合试验以评估胶质母细胞瘤细胞的迁移能力。简而言之,将细胞以1.0×105/孔的密度接种到六孔板中,直到它们达到80%的融合。用移液管尖端在融合的U87或U251细胞单层中产生划痕。在实验开始时,伤口的宽度被评估为相同。用PBS冲洗孔三次,以去除漂浮的细胞和碎片。为了测试紫草素对人胶质母细胞瘤细胞迁移的影响,将亲本U87或U251细胞接种在有或没有紫草素(2.5、5或7.5μmol/L)的无血清DMEM中。然后将这些细胞孵育0-48小时。培养板在37°C和5%CO2中孵育。在0、24和48小时时,使用相差显微镜测量伤口愈合情况,并随着时间的推移用照片记录。 体外侵袭试验[4] 如前所述,使用具有8-μm孔径插入物的Transwell侵袭试验检查了紫草素对人胶质母细胞瘤细胞侵袭的影响。Transwell过滤器插入物的膜上涂有以1:7的比例用介质稀释的Matrigel。如上所述制备亲本U87或U251细胞。将500微升补充有10%FBS的DMEM放入下腔室。无血清DMEM作为阴性对照。将紫草素(2.5、5或7.5μmol/L)、pIRES2-p-β-catenin、shRNA-p-β-calenin、LY294002(20μmol/L)或紫草素(5μmol/L)与IGF-1(20μg/mL,Proteintech:Chicago,IL,USA)联合加入上腔细胞悬浮液中。孵育0-48小时后,取出插入物,如上所述在显微镜下进行观察。在6个随机高倍视野(×400)中计数侵袭细胞的平均数量。 蛋白质印迹分析[4] 为了测定p-β-catenin的表达,进行了Western blot分析。U87或U251细胞用浓度为2.5、5和7.5μmol/L的Shikonin/紫草素处理48小时。用冰冷的PBS洗涤细胞三次以停止刺激。然后,收集细胞并在含有50 mmol/L Tris(PH 7.4)、150 mmol/L NaCl、1%Triton X-100、1%脱氧胆酸钠、0.1%SDS、1 mmol/L原钒酸钠、50 mmol/L氟化钠和1 mmol/L EDTA的冰冷放射免疫沉淀分析裂解缓冲液中裂解30分钟。然后用超声波破碎机破碎沉淀物,在4°C下以17000 rpm离心样品60分钟。收集上清液作为可溶性部分,并转移到新的试管中。立即在沸水浴中加热样品管5分钟以使蛋白质变性。用BCA蛋白测定试剂盒测定可溶性物质的蛋白质浓度。 |
| 动物实验 |
Intestinal Motility Measurement [1]
ICR mice were starved for 24 h and then orally administered Shikonin (5.8 μg). After 30 min, 20 mg of 10% activated charcoal diluted in 5% gum arabic was administered orally. After another 30 min, the animals were sacrificed and the small intestines were removed. Peristaltic index was calculated as the ratio of the length that activated charcoal had traveled to the total length of the small intestine. Mouse Model of Rotaviral Diarrhea [1] Neonatal ICR mice (age 4–7 days, weight 2–3 g) were inoculated with 30 μL rotavirus (virus titer 1.2 × 107 pfu/mL) by oral gavage using a polyethylene tube (0.6 mm outer diameter, 0.3 mm inner diameter) and an insulin syringe. The mice were then returned to their mothers and allowed to suckle. Stool samples were collected daily by gentle palpation of the abdomen. In one set of the experiments, the Shikonin-treated group received Shikonin orally (0.4 and 1.7 μg in 30 μL PBS) the day before virus inoculation, and three times a day until day 3. Control mice received 30 μL PBS alone. The positive control (CaCCinh-A01) mice received 34 μg (in 20 μL PBS) CaCCinh-A01 by intraperitoneal injection on the day before virus inoculation and twice a day thereafter until day 3. In another set of experiments, shikonin-treated group received shikonin in PBS on the next day of virus inoculation, and three times a day until day 3. Negative control mice received 30 μL PBS alone. The positive control mice received CaCCinh-A01 by intraperitoneal injection the day before virus inoculation and twice a day thereafter until day 3. Chemically-induced mouse skin carcinogenesis [2] Sixty 6–8-week old female DBA/2 mice (which are relatively sensitive to skin carcinogenesis) were divided into 4 groups: DMSO, DMBA/TPA, SKN, SKN+DMBA/TPA. The DMSO group (5 mice) received DMSO treatment as the vehicle control; the DMBA/TPA group received a single topical application of 200 nmol DMBA for 2 weeks, following by a single topical application of 5 μg TPA (12-O-tetradecanoylphorbol-13-acetate), once per day, three times per week for 14 weeks. The SKN group received topical application of Shikonin at 10 μg following the same schedule for DMBA/TPA treatments. The SKN+DMBA/TPA groups received shikonin (SKN) treatment first followed by TPA treatment 30 min later. At the end of the skin carcinogenesis study, mice were euthanized by pentobarbital (150 mg/kg, i.p.). The skin samples from experimental sites were collected and submitted for biochemical and morphologic analysis as described in the following. Experimental groups and treatment [5] The rats were randomly assigned to three groups: Sham-operated group (n=10), osteoarthritis model group (n=10) and sShikonin-treated group (n=10). In the sham-operated group, the right knee joint of the anesthetized rat was only exposed under sterile conditions, and the rats were treated with 0.1 ml/100 g physiological saline (i.p.). In the osteoarthritis model group, osteoarthritis model rats were treated with 0.1 ml/100 g physiological saline (i.p.). In the shikonin-treated group, osteoarthritis model rats were treated with 10 mg/kg shikonin (i.p.) once daily for 4 days after osteoarthritis modeling (14,15). ELISA analysis [5] Following treatment with 10 mg/kg Shikonin, peripheral blood was collected from the abdominal aorta of rats in each group (n=10). The blood was centrifuged at 12,000 × g for 10 min at 4°C and the supernatant was analyzed for IL-1β, TNF-α and iNOS using ELISA assay kits according to the manufacturer's protocol (Beijing 4A Biotech Co., Ltd.). Western blot analysis [5] Following the treatment with 10 mg/kg Shikonin, rats were anesthetized with 50 mg/kg pentobarbital intraperitoneally (i.p.), sacrificed by decapitation, and samples of arthrotic tissue were collected (n=10 per group). The samples were homogenized with radioimmunoprecipitation assay (RIPA) lysis buffer. The homogenate was centrifuged at 12,000 × g for 10 min at 4°C and analyzed using a bicinchoninic acid (BCA) assay kit. Approximately 50 µg protein was separated by electrophoresis on a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel and then transferred onto a nitrocellulose filter membrane. Proteins were detected using mouse anti-nuclear factor (NF)-κB p65 (sc-29311; 1:500), anti-cyclooxygenase (COX)-2 (sc-23984; 1:300), anti-Akt (sc-8312; 1:500) and anti-phosphorylated-Akt (anti-p-Akt; sc-135650; 1:1,000) and anti-β-actin (BB-2101-1; 1:5,000) followed by horseradish peroxidase-conjugated goat antimouse antibody (sc-2777; 1:5,000). The relative quantities of protein expression were measured using AlphaEase FC software. Caspase-3 activity analysis [5] Following the 4-day treatment with 10 mg/kg Shikonin, rats were sacrificed and osteoarthritis samples were collected. The samples were homogenized with RIPA lysis buffer. The homogenate was centrifuged at 12,000 × g for 10 min at 4°C and analyzed using a BCA assay kit. Protein (20 µg) was mixed with the substrate Ac-DEVD-pNA in reaction buffer, and incubated at 37°C for 2 h in the dark. The absorption was then detected at a wavelength of 405 nm. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Alkannin and shikonin are naturally occurring hydroxynaphthoquinones with a well-established spectrum of wound healing, antimicrobial, anti-inflammatory, and antioxidant activities. Recently, extensive scientific effort has been focused on their effectiveness on several tumors and mechanism(s) of antitumor activity. Liposomes have been proved as adequate drug carriers offering significant advantages over conventional formulations, such as controlled release and targeted drug delivery, leading to the appearance of several liposomal formulations in the market, some of them concerning anticancer drugs. The aim of the present study was to prepare shikonin-loaded liposomes for the first time in order to enhance shikonin therapeutic index. An optimized technique based on the thin film hydration method was developed and liposomes characterization was performed in terms of their physicochemical characteristics, drug entrapment efficiency, and release profile. Results indicated the successful incorporation of shikonin into liposomes, using both 1,2-dipalmitoylphosphatidylcholine and egg phosphatidylcholine lipids. Liposomes presented good physicochemical characteristics, high entrapment efficiency and satisfactory in vitro release profile. In vitro cytotoxicity of liposomes was additionally tested against three human cancer cell lines (breast, glioma, and non-small cell lung cancer) showing a moderate growth inhibitory activity. Practical applications: Shikonin is a naturally occurring hydroxynaphthoquinone and extensive scientific research (in vitro, in vivo, and clinical trials) has been conducted during the last years, focusing on its effectiveness on several tumors and mechanism(s) of antitumor action. The purpose of this work was to prepare and characterize shikonin-loaded liposomes as a new drug delivery system for shikonin. Liposomal formulations provide significant advantages over conventional dosage forms, such as controlled release and targeted drug delivery for anticancer agents. Thus, liposomes could reduce shikonin's side effects, enhance selectivity to cancer cells and protect shikonin from internal biotransformations and instability matters (oxidization and polymerization). Furthermore, liposomal delivery helps overcome the low aqueous solubility of shikonin, which is the major barrier to its oral and internal administration, since it cannot be dissolved and further absorbed from the receptor. Pharmacokinetics study has shown that shikonin absorption is fast if given by oral gavage and muscle injection, as it is barely detected in the plasma after 1 min, with a oral gavage yielding a bioavailability of about 34% (Wang et al., 1988). In this study, the doses used for intestinal motility (0.38 mg/kg) and rotaviral infection in the mice model (0.69 mg/kg) were below the doses that are considered as toxic.[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Reduction of stool water content in rotaviral neonatal mice by shikonin observed in this study probably occurred via an anti-secretory action of shikonin, which involved inhibition of CaCCGI chloride channel activity. Although TMEM16A exists in enterocytes, some investigators have proposed that secretory diarrhea caused by the rotaviral non-structural protein NSP4 is predominantly through the activation of epithelial TMEM16A in the intestine. One study that used the small molecule TMEM16A inhibitor T16Ainh-A01 has demonstrated that TMEM16A constitutes only a minor component of the intestinal epithelial CaCC (Namkung et al., 2011). Our previous work has shown that TMEM16A and CaCCGI have different characteristics, since the lignan compounds kobusin and eudesmin can affect TMEM16A and CaCCGI differently, inhibiting TMEM16A, while activating CaCCGI, (Jiang et al., 2015). We have shown in this study that shikonin was inhibitory toward CaCCGI-mediated short-circuit currents in both cell culture model and isolated mouse colon. In addition, in vivo studies showed that shikonin reduced water content in a neonatal mice diarrhea model without affecting the rotavirus infection process (Figure 6). These findings supported the view that the primary pathway of watery diarrhea associated with rotaviral infection is through activation of CaCCGI rather than TMEM16A by NSP4, thereby enhancing the accumulation of fluid. Furthermore, inhibition of TMEM16A by shikonin delayed gastrointestinal motility, helping to prolong the fluid absorption time to further decrease net fluid secretion.
Despite the many positive benefits of shikonin, it is not without toxicity. Intraperitonal injection of shikonin has been demonstrated to result in some toxicity, with an LD50 of 20 mg/kg (Sankawa et al., 1977). Pharmacokinetics study has shown that shikonin absorption is fast if given by oral gavage and muscle injection, as it is barely detected in the plasma after 1 min, with a oral gavage yielding a bioavailability of about 34% (Wang et al., 1988). In this study, the doses used for intestinal motility (0.38 mg/kg) and rotaviral infection in the mice model (0.69 mg/kg) were below the doses that are considered as toxic.[1] 72521 rat LD50 oral >1 gm/kg Nahrung. Chemistry, Biochemistry, Microbiology, Technology, Nutrition., 15(505), 1971 [PMID:5172860] 72521 mouse LD50 oral 3 gm/kg Nahrung. Chemistry, Biochemistry, Microbiology, Technology, Nutrition., 15(505), 1971 [PMID:5172860] Antidote and Emergency Treatment /SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/ /SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/ /SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/ Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3Rd edition, Elsevier Mosby, St. Louis, MO 2005, p. 160-1 Human Toxicity Excerpts /ALTERNATIVE and IN VITRO TESTS/ Shikonin has the potential to prevent, or be used in the treatment of bladder transitional cell carcinoma induced by arylamines. /Investigators/ evaluated its effectiveness by measuring the amount of acetylated 2-aminofluorene (AF), AF-DNA adducts, changes of / N-acetyltransferase (NAT)/ mRNA and the amount of NAT enzyme. T24 human bladder cancer cells were incubated with 30 uM AF with different concentrations of shikonin for various times. T24 cells treated with shikonin (16 uM) were then harvested and used in 2 experiments: 1). T24 cells were incubated with 22.5 uM AF and shikonin (0, 16 uM) (co-treatment) for 6, 12, 18, 24 and 48 hr). T24 cells were incubated with various concentrations of AF and shikonin (0, 16 uM) for 24 hr AF and AAF were measured by HPLC. Then in the prepared human T24 cell cytosols different concentrations of AF and shikonin were added to measure the kinetic constants of NAT. Next, AF-DNA adducts in human T24 cells with or without treatment with shikonin were detected and measured. The final two steps included measuring the NAT Ag-Ab complex after treatment with and without shikonin and evaluating the effect of shikonin on the NAT genes. Higher concentrations of shikonin induced decreasing AF acetylation. /It was/ found that the longer the culture period, the greater the difference in AF acetylation in the same shikonin concentrations. It was also noted that increase in AAF was proportional to incubation time. In the presence of 16 uM of shikonin, N-acetylation of AF decreased by up to 72-84%. Shikonin decreased the amount of AAF production in human T24 cells in all examined AF doses. Both Km and Vmax values in the cytosolic NAT decreased after the addition of shikonin to the cytosol. Finally, shikonin decreased the amount of AAF production and AF-DNA adducts formation in human 724 cells in all examined AF doses. The percentage of cells stained by antibody was significantly different after treatment with shikonin, especially with the higher shikonin concentrations. The NAT1 mRNA level and the NAT1/beta-actin ratio decreased significantly with higher concentrations (16-24 uM) of shikonin. Shikonin affected NAT activity, gene expression (NAT1 mRNA), AF-DNA adducts formation and formation of NAT Ag-Ab in human bladder tumor T24 cells... PMID:15011747 /ALTERNATIVE and IN VITRO TESTS/ Shikonin isolated from the roots of the Chinese herb Lithospermum erythrorhizon has been associated with anti-inflammatory properties. /Investigators/ evaluated shikonin's chemotherapeutic potential and investigated its possible mechanism of action in a human cutaneous neoplasm in tissue culture. Shikonin preferentially inhibits the growth of human epidermoid carcinoma cells concentration- and time-dependently compared to SV-40 transfected keratinocytes, demonstrating its anti-proliferative effects against this cancer cell line. Additionally, shikonin decreased phosphorylated levels of EGFR, ERK1/2 and protein tyrosine kinases, while increasing phosphorylated JNK1/2 levels. Overall, shikonin treatment was associated with increased intracellular levels of phosphorylated apoptosis-related proteins, and decreased levels of proteins associated with proliferation in human epidermoid carcinoma cells. PMID:14568164 /ALTERNATIVE and IN VITRO TESTS/ This study investigated the potential of shikonin as an anticancer agent against liver cancer and an in vitro human hepatoma cancer model system. The HepG2 cell line was the hepatoma cancer model in the present study. The inhibitory effect of shikonin on the growth of HepG2 cells was measured by MTT assay. To explore the underlying mechanism of cell growth inhibition of shikonin, the cell cycle distribution, DNA fragmentation, mitochondrial membrane potential disruption, and expression of Bax and Bcl-2 were measured in HepG2 cells. The activity of shikonin in inducing apoptosis was investigated through the detection of Annexin V signal and CD95 expression by flow cytometry and electron microscopy, respectively. Shikonin inhibited the growth of HepG2 cells in a dose-dependent manner. The IC50 value (inhibiting cell growth by 50%) was 4.30 mg/mL. Shikonin inhibited cell growth in a dose-dependent manner and blocked HepG2 cell cycle progression at the S phase. The changes in mitochondrial morphology, dose-dependently decreased in mitochondrial membrane potential, were observed in different concentrations of the drug treatment group. Western blot analysis showed that cajanol inhibited Bcl-2 expression and induced Bax expression. ...shikonin increases Annexin V signal and CD95 (Fas/APO) expression, resulting in apoptotic cell death of HepG2 cells. In addition, lump formation of intranuclear chromatin, pyknosis of cell nucleus, deletion of microvillus, vacuolar degeneration of mitochondria, reduction of rough endoplasmic reticulum, and resolution of free ribosome, etc., associated with apoptosis were discovered by electron microscopy in HepG2 cells after 48 hr treatment. Shikonin inhibited HepG2 cells, possibly through the pathway of inducing early apoptosis, and was beneficial for restoring the apoptotic sensitivity of HepG2 cells by CD95, and should therefore be considered as a candidate agent for the prevention or treatment of human hepatoma. PMID:21164560 /ALTERNATIVE and IN VITRO TESTS/ Shikonin (SK) has been isolated and identified as a key bioactive component in an herbal plant, Shikon (gromwell). /This study/ investigated antiestrogen activity of SK in breast cancer cells /MCF-7, T47D and MDA-MB-231 cells/. In human breast cancer cells, we observed that treatment with SK inhibits tumor cell growth in estrogen receptor alpha (ERalpha)-positive, but not ERalpha-negative breast cancer cells. Estrogen-dependent cell growth was inhibited by co-treatment with SK. A potential molecular mechanism by which SK inhibits estrogen action was explored... SK has no effect on ERalpha mRNA expression, but decreases its protein level. This effect is associated with an increase in ubiquitinated ERalpha for degradation. /The/ results suggest that SK downregulates ERalpha protein through a proteasome-mediated pathway. ...treatment with SK inhibits estrogen-induced estrogen response elements reporter gene activity. Furthermore, SK inhibits recruitment of ERalpha at the estrogen-dependent gene promoters, and subsequently suppresses gene expression. Finally, co-treatment with SK enhanced sensitivity of breast cancer cells to endocrine therapy... PMID:19760501 Non-Human Toxicity Excerpts /LABORATORY ANIMALS: Subchronic or Prechronic Exposure/ /The objective of this study was / to investigate the anti-inflammatory or immunomodulatory effect of shikonin on early stage and established murine collagen-induced arthritis (CIA). /Mice/ were injected intraperitoneally with shikonin (5 mg/kg) for 10 days along, before, or after the onset of CIA. The arthritis response was monitored visually by macroscopic scoring. Reverse transcription-polymerase chain reaction and western blotting were employed to determine the mRNA and protein expression of cytokine in patella with adjacent synovium in CIA /mice/. Histology of knee was used to assess the occurrence of cartilage destruction and bone erosion. Shikonin (5 mg/kg) treatment along had no effect on macroscopic score and incidence of arthritis on early stage of CIA. However, a pronounced amelioration of macroscopic score and cartilage destruction was found in mouse treated with shikonin on established CIA for 10 days. Moreover, The mRNA levels of Th1 cytokines [tumor necrosis factor-alpha and interleukin (IL)-12] was significantly inhibited both in the synovial tissue and in the articular cartilage in treated groups compared with those in control groups, whereas the mRNA and protein levels of Th2 cytokines (IL-10 and IL-4) remained elevated throughout the treatment period. Moreover, the inflammatory cytokine, the mRNA and protein levels of IL-6 were down-regulated in mice with established CIA after treatment with shikonin. T-box expressed in T cells (T-bet) mRNA levels were decreased in shikonin compared with control group, and GATA-3 mRNA levels were higher than that in control group. Shikonin treatment on established CIA can inhibit Th1 cytokines expression and induce Th2 cytokines expression in mice with established CIA. The inhibited effect of shikonin on Th1 cytokines expression may be mediated not only by inhibiting Th1 responses through T-bet mechanism, but also by inducing anti-inflammatory mediators such as IL-10 and IL-4 through a GATA-3 dependent mechanism. PMID:18781399 |
| 参考文献 | |
| 其他信息 |
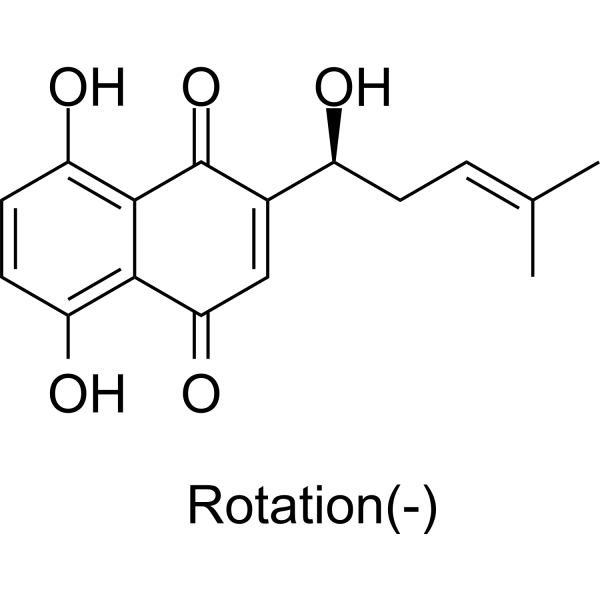
Alkannin is a hydroxy-1,4-naphthoquinone.
Alkannin has been reported in Arnebia decumbens, Arnebia euchroma, and other organisms with data available. Shikonin is a hydroxy-1,4-naphthoquinone. Shikonin has been reported in Arnebia decumbens, Arnebia euchroma, and other organisms with data available. See also: Arnebia guttata root (part of); Arnebia euchroma root (part of); Lithospermum erythrorhizon root (part of). Mechanism of Action /Investigators/ previously developed a gene-gun-based in vivo screening system and identified shikonin as a potent suppressor of tumor necrosis factor-alpha (TNF-alpha) gene expression. Here... shikonin selectively inhibits the expression of TNF-alpha at the RNA splicing level. Treatment of lipopolysaccharide-stimulated human primary monocytes and THP-1 cells with shikonin resulted in normal transcriptional induction of TNF-alpha, but unspliced pre-mRNA accumulated at the expense of functional mRNA. This effect occurred with noncytotoxic doses of shikonin and was highly specific, because mRNA production of neither a housekeeping gene nor another inflammatory cytokine gene, interleukin-8 (IL-8), was affected. Moreover, cotreatment with lipopolysaccharide (LPS) and shikonin increased the endpoint protein production of IL-8, accompanied by suppressed activation of the double-stranded RNA-activated protein kinase (PKR) pathway. Because PKR inactivation has been shown to down-regulate the splicing process of TNF-alpha RNA and interfere with translation, our findings suggest that shikonin may achieve differential modulation of cytokine protein expression through inactivation of the PKR pathway and reveal that regulation of TNF-alpha pre-mRNA splicing may constitute a promising target for future anti-inflammatory application. Shikonin isolated from the roots of the Chinese herb Lithospermum erythrorhizon has been associated with anti-inflammatory properties. /Investigators/ evaluated shikonin's chemotherapeutic potential and investigated its possible mechanism of action in a human cutaneous neoplasm in tissue culture. Shikonin preferentially inhibits the growth of human epidermoid carcinoma cells concentration- and time-dependently compared to SV-40 transfected keratinocytes, demonstrating its anti-proliferative effects against this cancer cell line. Additionally, shikonin decreased phosphorylated levels of EGFR, ERK1/2 and protein tyrosine kinases, while increasing phosphorylated JNK1/2 levels. Overall, shikonin treatment was associated with increased intracellular levels of phosphorylated apoptosis-related proteins, and decreased levels of proteins associated with proliferation in human epidermoid carcinoma cells. ... /A previous study showed/ that shikonin, a natural compound isolated from Lithospermun erythrorhizon Sieb. Et Zucc, inhibits adipogenesis and fat accumulation. This study was conducted to investigate the molecular mechanism of the anti-adipogenic effects of shikonin. Gene knockdown experiments using small interfering RNA (siRNA) transfection were conducted to elucidate the crucial role of beta-catenin in the anti-adipogenic effects of shikonin. Shikonin prevented the down-regulation of beta-catenin and increased the level of its transcriptional product, cyclin D1, during adipogenesis of 3T3-L1 cells, preadipocytes originally derived from mouse embryo. beta-catenin was a crucial mediator of the anti-adipogenic effects of shikonin, as determined by siRNA-mediated knockdown. Shikonin-induced reductions of the major transcription factors of adipogenesis including peroxisome proliferator-activated receptor gamma and CCAAT/enhancer binding protein alpha, and lipid metabolizing enzymes including fatty acid binding protein 4 and lipoprotein lipase, as well as intracellular fat accumulation, were all significantly recovered by siRNA-mediated knockdown of beta-catenin. Among the genes located in the WNT/beta-catenin pathway, the levels of WNT10B and DVL2 were significantly up-regulated, whereas the level of AXIN was down-regulated by shikonin treatment. This study ...shows that shikonin inhibits adipogenesis by the modulation of WNT/beta-catenin pathway in vitro, and also suggests that WNT/beta-catenin pathway can be used as a therapeutic target for obesity and related diseases using a natural compound like shikonin... As the major component of Zicao, the dried root of Lithospermum erythrorhizon, shikonin has been broadly used due to its anti-inflammatory activity (Chen et al., 2002). Shikonin has been reported to have antioxidant, antibacterial, antiparasitic, antiviral and wound-healing activities (Andujar et al., 2013). Shikonin might be used in the treatment of asthma. Takano-Ohmuro et al. (2008) has explored the use of shikonin in asthma by focusing on its anti-inflammatory activity (Takano-Ohmuro et al., 2008). Other investigators have used a mouse asthma model to demonstrate that shikonin inhibits bone marrow-derived dendritic cell maturation in vitro, and allergic action as well as tracheal hyperresponsiveness in vivo (Lee et al., 2010). Since TMEM16A is expressed in airway smooth muscle cells and it participates in smooth muscle contraction (Huang et al., 2012), we assumed that TMEM16A may be involved in shikonin-mediated inhibition of asthma. In our study, shikonin was found to inhibit TMEM16A chloride channel activity, indicating that shikonin might alleviate asthma by inhibiting smooth muscle contraction in the trachea.[1] In summary, the results from this study suggest that shikonin is effective in inhibiting chemically-induced skin carcinogenesis which is mediated largely by inhibiting cell proliferation during skin carcinogenesis. The potential target identified in this study, ATF2, will be examined in future experiments.[2] In conclusion, our findings indicated that shikonin promoted proliferation of skin cells by a currently unknown mechanism, while shikonin did not induce COL1 expression in HDFs. In addition, shikonin inhibited NF-κB signaling pathway and proteasome activity in HDFs, which suggested an anti-inflammatory effect of shikonin. Therefore, shikonin may be a potential therapeutic agent both in wound healing and in the treatment of inflammatory skin diseases. Thus, shikonin may be most suitable in the treatment of refractory inflammatory skin ulcers.[3] Shikonin attenuates the proliferation, migration, and invasion capability of human glioblastoma cells by inhibiting MMP-2, MMP-9. In p53 wild-type glioma cells, the mechanism is associated with downregulated phosphorylated β-catenin Y333 and p-PI3K/p-Akt expression. In p53 mutant glioma cells, it correlates to an inhibited PI3K/Akt pathway.[4] In conclusion, the results of the present study confirmed that shikonin inhibits inflammation and chondrocyte apoptosis by regulating the PI3K/Akt signaling pathway in a rat model of osteoarthritis. These findings suggest that shikonin has therapeutic potential for osteoarthritis.[5] |
| 分子式 |
C16H16O5
|
|---|---|
| 分子量 |
288.2952
|
| 精确质量 |
288.099
|
| 元素分析 |
C, 66.66; H, 5.59; O, 27.75
|
| CAS号 |
517-88-4
|
| 相关CAS号 |
Shikonin;517-89-5;Alkannin;23444-65-7
|
| PubChem CID |
72521
|
| 外观&性状 |
Brown to red solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
567.4±50.0 °C at 760 mmHg
|
| 熔点 |
149°
|
| 闪点 |
311.0±26.6 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.642
|
| LogP |
4.35
|
| tPSA |
94.83
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
501
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CC(=CC[C@@H](C1=CC(=O)C2=C(C=CC(=C2C1=O)O)O)O)C
|
| InChi Key |
NEZONWMXZKDMKF-JTQLQIEISA-N
|
| InChi Code |
InChI=1S/C16H16O5/c1-8(2)3-4-10(17)9-7-13(20)14-11(18)5-6-12(19)15(14)16(9)21/h3,5-7,10,17-19H,4H2,1-2H3/t10-/m0/s1
|
| 化学名 |
5,8-dihydroxy-2-[(1S)-1-hydroxy-4-methylpent-3-enyl]naphthalene-1,4-dione
|
| 别名 |
(-)-Alkannin; 517-88-4; Anchusin; Alkanna Red; Anchusa acid; Alkannin (VAN); Anchusin (VAN); Alkanna red (VAN);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~33.33 mg/mL (~115.61 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.67 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.67 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4686 mL | 17.3430 mL | 34.6861 mL | |
| 5 mM | 0.6937 mL | 3.4686 mL | 6.9372 mL | |
| 10 mM | 0.3469 mL | 1.7343 mL | 3.4686 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|