| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
PKA; PDE
In vitro activity: Bucladesine sodium (also known as Dibutyryl-cAMP) is a cell-permeable PKA activator and a cAMP analog that mimics the action of endogenous cAMP. It is a cyclic nucleotide derivative (structurally similar to cAMP) and is also a phosphodiesterase inhibitor. Dibutyryl-cAMP preferentially activates cAMP-dependent protein kinase. The products releaes butyrate due to intracellular and extracellular esterase action. Butyrate was shown to have distinct biological effects. The compound is used in a wide variety of research applications because it mimics cAMP and can induce normal physiological responses when added to cells in experimental conditions. Kinase Assay: Bucladesine sodium (also known as Dibutyryl-cAMP) is a cell-permeable PKA activator and a cAMP analog that mimics the action of endogenous cAMP. It is a cyclic nucleotide derivative (structurally similar to cAMP) and is also a phosphodiesterase inhibitor. Dibutyryl-cAMP preferentially activates cAMP-dependent protein kinase. |
|---|---|
| 体外研究 (In Vitro) |
体外活性:布克拉地辛钠(也称为二丁酰-cAMP)是一种细胞渗透性 PKA 激活剂和模拟内源性 cAMP 作用的 cAMP 类似物。它是一种环核苷酸衍生物(结构与cAMP相似),也是一种磷酸二酯酶抑制剂。二丁酰-cAMP 优先激活 cAMP 依赖性蛋白激酶。该产品由于细胞内和细胞外酯酶的作用而释放丁酸盐。丁酸盐被证明具有独特的生物效应。该化合物可用于多种研究应用,因为它模仿 cAMP,并且在实验条件下添加到细胞中时可以诱导正常的生理反应。激酶测定:布克拉地辛钠(也称为二丁酰-cAMP)是一种细胞渗透性 PKA 激活剂和模拟内源性 cAMP 作用的 cAMP 类似物。它是一种环核苷酸衍生物(结构与cAMP相似),也是一种磷酸二酯酶抑制剂。二丁酰-cAMP 优先激活 cAMP 依赖性蛋白激酶。
研究开发了一种用于局部给药的含 Bucladesine (DB-cAMP) 的无水乳剂。测定了 Bucladesine (DB-cAMP) 在不同溶剂中的溶解度。其在疏水性溶剂中溶解度差,但在丙二醇、乙二醇和N-甲基吡咯烷酮中溶解度良好。该化合物溶解在N-甲基吡咯烷酮中时保持稳定,14天内未观察到水解。[3] |
| 体内研究 (In Vivo) |
雄性大鼠双侧输注 10 mM 或 100 mM 布克拉地辛,结果显示与对照组相比,逃避潜伏期和行走距离显着缩短(表明空间记忆得到改善)。雄性大鼠在接受 0.5 mg 尼古丁后几分钟内注射 1 或 5 mM 布氯地辛,保留空间记忆的能力会增强 [1]。当布克拉地辛(10 mM/侧)和尼古丁(0.5 mM/侧)联合使用时,CA1 区域的 ChAT 和 VAChT 免疫反应性显着升高。此外,使用尼古丁和低剂量布克拉地辛治疗时,ChAT 和 VAChT 免疫染色的光密度和数量显着增加。大鼠的逃避延迟和行走距离减少[2]。将水溶液施用于皮肤切除部位后,布克拉地辛几乎很快被完全吸收。与全层磨损大鼠模型相比,在没有皮肤脱皮的情况下,布克拉地辛的吸收更慢但更快[3]。在花生四烯酸引起的小鼠耳水肿模型中,布克拉地辛(含1.5%乳剂,单次或多次注射)可显着减轻炎性水肿[4]。
将3%的 布卡地辛 水溶液 (30 mg/只) 局部涂抹于皮肤完整的大鼠后,血浆浓度极低,系统生物利用度仅为 1.2 ± 0.5%。 [2] 将相同的3%水溶液涂抹于全层皮肤擦伤部位时,布卡地辛 吸收非常迅速 (达峰时间 0.5 小时),且几乎被完全吸收(生物利用度:93.1 ± 34.3%)。 [2] 将3%水溶液涂抹于剥离了角质层的皮肤后,吸收也很快(达峰时间 0.5 小时),生物利用度为 63.5 ± 14.4%,但峰值血药浓度约为全层擦伤模型的四分之一。 [2] 3%的 布卡地辛 聚乙二醇软膏能提供持续释放。涂抹于全层擦伤部位时,生物利用度为 31.8 ± 27.4%,与水溶液相比达峰时间延迟。涂抹于剥离角质层的皮肤时,PEG软膏的生物利用度为 10.6 ± 4.1%。 [2] 3%的 布卡地辛 凡士林软膏在剥离角质层(生物利用度:0.6 ± 1.4%)和全层擦伤模型(生物利用度:3.7 ± 0.7%)中的吸收均非常低。 [2] 将 布卡地辛 原料药粉末 (30 mg) 局部涂抹于剥离角质层的皮肤后,吸收迅速且几乎完全(生物利用度:84.3 ± 18.4%),峰值血药浓度高于水溶液。 [2] |
| 酶活实验 |
PKA试验[4]
用10 mM磷酸钠缓冲液,pH 7.4, 0.15 M NaC1洗涤细胞2次,然后用1 ml相同的缓冲液从培养板上刮下。离心收集细胞,在细胞匀浆缓冲液(50 mMTris-HC1, pH 7.4, 1 mM EDTA, 1 mM二硫苏糖醇(DTT), 50 mM胰肽,0.1 mM苯基甲基磺酰氟)中进行短暂超声匀浆。在4°c, 14000 rpm的微离心机中离心20 mm,去除颗粒部分。采用froskoski(1983)的方法,使用合成肽底物Leu-Arg-ArgAla-Ser-Leu-Gly (Kemptide),在上清液中测量PKA活性。反应混合物为50 ~。含有细胞裂解液,终浓度为25 mM Tris-HC1缓冲液(pH7.4), 5 mM醋酸镁,5 mM DTT, 5 mM cAMP, 20,~iMKemptide, 0.25 mM异丁基甲基黄嘌呤,0.1 mM [y- 32P I ATP (200 cpm/pmol),当添加20,uM PKA肽抑制剂5-24时。在30°温度下,用50jtl的7.5 mm磷酸终止反应10 mm。将50微升反应混合物放在P81过滤器上,用75 mM磷酸洗涤5次,并按前面描述的计数。PKA肽抑制剂5-24存在与不存在时的活性差异用于计算PKA活性。 PKC检测[4] 按照pka实验的描述制备细胞裂解物。反应液为50 j.el,终浓度为25 mM Tris-HC1缓冲液(pH 7.4), 5 mM醋酸镁,5 mM DTT, 20 ~。tM合成底物(Pro-Leu-Ser-Arg-Thr-Leu-Ser-Val-Ala-Ala-LysLys), 0.25 mM异丁基甲基黄嘌呤,0.1 mM [y32p] ATP (200 cpm/pmol)。反应在30°C下孵育10 mm,用磷酸终止,并按照PKA试验的描述进行分析。作为对照,特异性PKC肽抑制剂19-36,在20。用tM对细胞提取物的活性有90%以上的抑制作用。 |
| 细胞实验 |
囊泡乙酰胆碱转运体(VAChT)基因和胆碱乙酰转移酶(ChAT)基因构成胆碱能基因座。我们研究了环腺苷酸依赖性蛋白激酶(PKA)在大鼠嗜铬细胞瘤细胞系PC12和PC12 PKA缺陷突变体中对这些基因的协同调节。二丁基环腺苷酸(dbcAMP)处理PC12细胞后,ChAT和VAChT mRNA均增加了约四倍。dbcAMP也能提高ChAT和PKA的活性。PKA缺陷细胞系中ChAT和VAChT mRNA的基础水平均比野生型PC12细胞低约6倍,并且通过添加dbcAMP诱导不到两倍。PKA的特异性抑制剂H-89和H-9将ChAT和VAChT mRNA水平降低到未处理细胞的约三分之一,ChAT活性降低到未治疗PC12细胞的约四分之一。激活PKA II型而不是PKA I型,使ChAT活性增加约三倍。报告基因构建体的分析表明PKA影响胆碱能基因位点上游位点的基因转录。这些结果表明,ChAT和VAChT基因的表达在转录水平上受到协同调节,特异性涉及PKA II的信号通路在这一过程中发挥着重要作用[5]。
|
| 动物实验 |
For topical administration of bucladesine as 5% solution, 20 μl of drug or vehicle solution was administered onto the outer surface of both, left and right ears each, 60 min prior to arachidonic acid challenge. The inflammatory response was induced by administration of 20 μl arachidonic acid (Sigma-Aldrich, Munich, Germany; 5% in acetone) on the outer surface of left ears. The right ears were treated with acetone only to determine the individual differences in ear thicknesses.Na ve male Albino Swiss mice
Percutaneous Absorption Study in Rats: Male Sprague-Dawley rats (8 weeks old) were used. Abdominal hair was shaved 16 hours prior to the experiment. Rats were anesthetized and fixed. Three skin conditions were created: 1) Normal intact skin. 2) Stripped skin: The stratum corneum was removed by repeated adhesive tape stripping (20 times). 3) Full-thickness abrasion: The abdominal skin was excised with scissors. A plastic cell (internal diameter 3 cm) was affixed to the application site. Buchadesine (30 mg per rat) was applied as a 3% aqueous solution, a 3% PEG ointment, a 3% petrolatum ointment, bulk powder, or various powder formulations (PW-1, PW-2, PW-3). The cell was sealed. Blood samples were collected from the jugular vein at 0.5, 1, 2, 4, and 8 hours after application. Plasma was separated and stored frozen until analysis. [2] Intravenous Administration for Pharmacokinetic Parameters: Rats were given a single intravenous dose of 3 mg Buchadesine as an aqueous solution. Blood samples were collected periodically for 8 hours to determine baseline pharmacokinetic parameters. [2] |
| 药代性质 (ADME/PK) |
After a single intravenous dose (3 mg) in rats, the plasma concentration of Buchadesine declined in a monoexponential manner. [2]
The elimination rate constant (ke) was 8.24 ± 1.25 h⁻¹. [2] The biological half-life was 5.14 ± 0.81 minutes. [2] The absolute bioavailability after topical application was calculated as the ratio of the area under the plasma concentration-time curve (AUC) for the transdermal route to the AUC after intravenous administration. [2] For a 3% aqueous solution applied to normal skin, stripped skin, and full-thickness abrasion, the bioavailabilities were 1.2 ± 0.5%, 63.5 ± 14.4%, and 93.1 ± 34.3%, respectively. [2] For a 3% PEG ointment applied to normal skin, stripped skin, and full-thickness abrasion, the bioavailabilities were 3.2 ± 0.8%, 10.6 ± 4.1%, and 31.8 ± 27.4%, respectively. [2] For a 3% petrolatum ointment applied to normal skin, stripped skin, and full-thickness abrasion, the bioavailabilities were 0.0 ± 0.1%, 0.6 ± 1.4%, and 3.7 ± 0.7%, respectively. [2] For bulk powder and formulated powders (PW-1, PW-2, PW-3) applied to stripped skin, the bioavailabilities were 84.3 ± 18.4%, 13.4 ± 8.1%, 2.9 ± 1.9%, and 13.7 ± 7.3%, respectively. [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
The literature states that Bucladesine (DB-cAMP) has an excellent safety profile based on its prior clinical use as a topical treatment for wound healing. No PDE4 inhibitor-like adverse effects (e.g., gastrointestinal effects such as nausea) are known from its clinical use. However, a challenge associated with its formulation is the potential generation of butyric acid due to hydrolysis, which causes an unpleasant odor. The water-free emulsion developed in this study aimed to prevent hydrolysis and was found to be stable without signs of hydrolysis or increased odor during the observation period. [3]
|
| 参考文献 |
|
| 其他信息 |
Buchadesine (sodium N⁶,2′-O-dibutyryl cyclic 3′,5′ adenosine monophosphate, DBcAMP) is effective for the treatment of chronic skin ulcers, including decubitus ulcers. [2]
The study demonstrates that the percutaneous absorption of Buchadesine is negligible through intact skin but significant through damaged skin, which is appropriate for its intended use on ulcers. [2] The formulation vehicle profoundly influences the rate and extent of absorption through damaged skin. PEG ointment provides a sustained release profile, which is desirable to minimize potential systemic adverse effects from high peak concentrations. [2] The results support the clinical observation that PEG ointment may produce better therapeutic effects on decubitus ulcers than petrolatum ointment, due to its more favorable drug release characteristics. [2] |
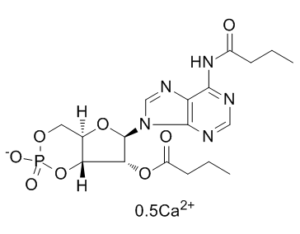
| 分子式 |
C18H24CAN5O8P
|
|
|---|---|---|
| 分子量 |
509.463624000549
|
|
| 精确质量 |
976.219
|
|
| CAS号 |
938448-87-4
|
|
| 相关CAS号 |
Bucladesine sodium;16980-89-5; 362-74-3
|
|
| PubChem CID |
44514776
|
|
| 外观&性状 |
White to off-white solid
|
|
| tPSA |
328Ų
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
22
|
|
| 可旋转键数目(RBC) |
16
|
|
| 重原子数目 |
65
|
|
| 分子复杂度/Complexity |
751
|
|
| 定义原子立体中心数目 |
8
|
|
| SMILES |
CCCC(=O)NC1=C2C(=NC=N1)N(C=N2)[C@H]3[C@@H]([C@H]4[C@H](O3)COP(=O)(O4)[O-])OC(=O)CCC.CCCC(=O)NC1=C2C(=NC=N1)N(C=N2)[C@H]3[C@@H]([C@H]4[C@H](O3)COP(=O)(O4)[O-])OC(=O)CCC.[Ca+2]
|
|
| InChi Key |
DRYMTGFYEAYJQR-NGVPHMJWSA-N
|
|
| InChi Code |
InChI=1S/2C18H24N5O8P.Ca/c2*1-3-5-11(24)22-16-13-17(20-8-19-16)23(9-21-13)18-15(30-12(25)6-4-2)14-10(29-18)7-28-32(26,27)31-14;/h2*8-10,14-15,18H,3-7H2,1-2H3,(H,26,27)(H,19,20,22,24);/t2*10-,14-,15-,18-;/m11./s1
|
|
| 化学名 |
calcium;[(4aR,6R,7R,7aR)-6-[6-(butanoylamino)purin-9-yl]-2-oxido-2-oxo-4a,6,7,7a-tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-7-yl] butanoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.12 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.12 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.12 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO +ddH2O: 30 mg/mL 配方 5 中的溶解度: 110 mg/mL (225.22 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9629 mL | 9.8143 mL | 19.6286 mL | |
| 5 mM | 0.3926 mL | 1.9629 mL | 3.9257 mL | |
| 10 mM | 0.1963 mL | 0.9814 mL | 1.9629 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Anti-inflammatory effect of 0.5 and 1.5% bucladesine cream given(a)3h before administration of arachidonic acid or given(b)twice, i.e., 7 and 3h before administration of arachidonic acid.Arch Dermatol Res.2012 May;304(4):313-7. |
Anti-inflammatory effect of 5% bucladesine given 1h before administration of arachidonic acid.Arch Dermatol Res.2012 May;304(4):313-7. |
Anti-inflammatory effect of 2.5% ketoprofen gel given 3h before administration of arachidonic acid.Arch Dermatol Res.2012 May;304(4):313-7. |