| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|

| 靶点 |
thrombin (Ki = 4.5 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:BIBR 953 是一种非常有效的抗凝血剂。 BIBR 953 表明末端苯基可以被更亲水的 2-吡啶基取代,而不会显着损失活性。 BIBR 953 抑制凝血酶、纤溶酶、因子 Xa、胰蛋白酶、tPA 和活化蛋白 C,Ki 分别为 4.5 nM、1.7 μM、3.8 μM、50 nM、45 μM 和 20 μM。 BIBR 953 特异性且可逆地抑制凝血酶。
|
| 体内研究 (In Vivo) |
BIBR 953 在对大鼠进行静脉注射后表现出最有利的活性。达比加群酯口服给药后达比加群的生物利用度为7.2%。口服治疗后达比加群主要通过粪便排泄,静脉注射治疗后达比加群主要通过尿液排泄。达比加群的平均终末半衰期约为 8 小时。口服和静脉注射给药后,达比加群酰基葡萄糖醛酸苷分别占尿液中剂量的 0.4% 和 4%。
|
| 动物实验 |
Male rats (Wessler model)[3]
0.01, 0.03, 0.05 and 0.1 mg/kg Intravenous injection |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute bioavailability of dabigatran following oral administration of dabigatran etexilate is approximately 3 to 7%. Dabigatran etexilate is a substrate of the efflux transporter P-gp. After oral administration of dabigatran etexilate in healthy volunteers, Cmax occurs at 1 hour post-administration in the fasted state. Coadministration of Dabigatran with a high-fat meal delays the time to Cmax by approximately 2 hours but has no effect on the bioavailability of dabigatran; Dabigatran may be administered with or without food. The oral bioavailability of dabigatran etexilate increases by 75% when the pellets are taken without the capsule shell compared to the intact capsule formulation. Dabigatran capsules should therefore not be broken, chewed, or opened before administration. Dabigatran is approximately 35% bound to human plasma proteins. The red blood cell to plasma partitioning of dabigatran measured as total radioactivity is less than 0.3. The volume of distribution of dabigatran is 50 to 70 L. Dabigatran pharmacokinetics are dose proportional after single doses of 10 to 400 mg. Given twice daily, dabigatran's accumulation factor is approximately two. For more Absorption, Distribution and Excretion (Complete) data for Dabigatran (10 total), please visit the HSDB record page. Metabolism / Metabolites After oral administration, dabigatran etexilate is converted to dabigatran. The cleavage of the dabigatran etexilate by esterase-catalyzed hydrolysis to the active principal dabigatran is the predominant metabolic reaction. Dabigatran is not a substrate, inhibitor, or inducer of CYP450 enzymes. Dabigatran is subject to conjugation forming pharmacologically active acyl glucuronides. Four positional isomers, 1-O, 2-O, 3-O, and 4-O-acylglucuronide exist, and each accounts for less than 10% of total dabigatran in plasma. The pharmacokinetics and metabolism of the direct thrombin inhibitor dabigatran (BIBR 953 ZW, beta-alanine, N-((2-(((4-(aminoiminomethyl)phenyl)amino)methyl)-1-methyl-1H-benzimidazol-5-yl)carbonyl)-N-2-pyridinyl) were studied in 10 healthy males, who received 200 mg of (14)C-dabigatran etexilate (BIBR 1048 MS, the oral prodrug of dabigatran) or an i.v. infusion of 5 mg of (14)C-dabigatran. Radioactivity was measured in plasma, urine, and feces over 1 week. The metabolite pattern was analyzed by high-performance liquid chromatography with on-line radioactivity detection, and metabolite structures were elucidated by mass spectrometry. Dabigatran etexilate was rapidly converted to dabigatran, with peak plasma dabigatran concentrations being attained after approximately 1.5 hr ...The predominant metabolic reaction was esterase-mediated hydrolysis of dabigatran etexilate to dabigatran. Phase I metabolites accounted for The half-life of dabigatran in healthy subjects is 12 to 17 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Chronic therapy with dabigatran is associated with moderate ALT elevations (greater than 3 times the upper limit of normal) in 1.5% to 3% of patients, an overall rate which is slightly lower than with low molecular weight heparin and similar to the rates with warfarin. While case reports of clinically apparent liver injury due to dabigatran have not been published, several instances of ALT elevations with jaundice occurred during the large, prelicensure clinical trials of dabigatran. These cases were mild and self-limited, resolving completely once therapy was stopped. However, other causes of liver injury could not always be identified and the relationship of the injury to dabigatran therapy remains unclear. The clinical features of these cases were not described. In one large clinical trial, these unexplained cases of liver injury with bilirubin elevations occurred in approximately 1 in 2000 patients treated. In a subsequent case report, liver injury with jaundice and a mixed pattern of serum enzyme elevations arose within 4 weeks of starting dabigatran and resolved rapidly with its discontinuation. Immunoallergic and autoimmune features were not present. There have been multiple spontaneous reports of liver injury, some of which were fatal, made to WHO and FDA surveillance databases, but the relatedness of the episodes has not been clearly defined. Thus, clinically apparent liver injury with jaundice due to dabigatran occurs but is rare and typically mild and self-limited. Likelihood score: D (possible rare cause of clinically apparent liver injury). One reason why dabigatran was subjected to close scrutiny for evidence of hepatotoxicity was that the initial oral, direct thrombin inhibitor developed and evaluated in clinical trials was ximelagatran (zye" mel a gat' ran), which subsequently was found to be associated with rare but potentially severe cases of liver injury, typically arising after 1 to 6 months of treatment with a hepatocellular pattern of serum enzyme elevations and potentially severe and fatal course. Ximelagatran did not receive approval for use in the United States because of concerns about hepatotoxicity. After several further cases of clinically apparent hepatic injury were found in patients taking ximelagatran, it was also withdrawn from use in Europe. Risk of serum ALT elevations during ximelagatran therapy were later shown to be linked to HLA-DRB1*07 and DQA1*-02. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation In adults, less than 7% of dabigatran is absorbed orally in its prodrug form of dabigatran etexilate mesylate; dabigatran itself is not absorbed orally. Preliminary data from 2 individuals indicate that dabigatran is poorly excreted into breastmilk and unlikely to affect the breastfed infant. If the mother requires dabigatran, it is not a reason to discontinue breastfeeding. Because data are limited, monitor preterm or newborn infants for signs of bleeding. ◉ Effects in Breastfed Infants Samples of newborn and preterm infant blood spiked with of dabigatran in the concentrations found in breastmilk after a 220 mg dose of dabigatran etexilate indicate that no effect on coagulation would occur. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions The concomitant use of a CYP3A4 isoenzyme substrate (atorvastatin) and dabigatran did not have clinically relevant effects on the pharmacokinetics of either drug. Also, the concomitant use of a CYP2C9 substrate (diclofenac) and dabigatran did not have clinically relevant effects on the pharmacokinetics of either drug. Administration of rifampin for 7 days followed by a single dose of dabigatran resulted in decreases of 66 and 67% in dabigatran area under the plasma concentration-time curve (AUC) and peak plasma concentration, respectively. Within 7 days of rifampin discontinuance, dabigatran exposure approached levels expected without concurrent use of rifampin. Concomitant use should be avoided. Concomitant use of dabigatran with P-glycoprotein inhibitors may increase systemic exposure to dabigatran. While clinical data and pharmacokinetic studies indicate that concomitant use of dabigatran with certain P-glycoprotein inhibitors (i.e., amiodarone, clarithromycin, ketoconazole, quinidine, verapamil) does not necessitate dosage adjustments, the manufacturer states that these results should not be extrapolated to all P-glycoprotein inhibitors. Concomitant use of P-glycoprotein transport inhibitors and dabigatran in patients with renal impairment is expected to increase systemic exposure to dabigatran compared with that resulting from either factor alone. Reduction of dabigatran dosage should be considered in patients with moderate renal impairment (creatinine clearance of 30-50 mL/minute) who are receiving concomitant dronedarone or systemic ketoconazole. Concomitant use of dabigatran and P-glycoprotein transport inhibitors in patients with severe renal impairment (creatinine clearance of 15-30 mL/minute) should be avoided. For more Interactions (Complete) data for Dabigatran (20 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
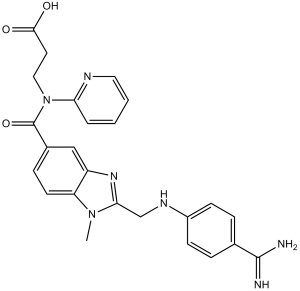
Dabigatran is an aromatic amide obtained by formal condensation of the carboxy group of 2-{[(4-carbamimidoylphenyl)amino]methyl}-1-methyl-1H-benzimidazole-5-carboxylic acid with the secondary amoino group of N-pyridin-2-yl-beta-alanine. The active metabolite of the prodrug dabigatran etexilate, it acts as an anticoagulant which is used for the prevention of stroke and systemic embolism. It has a role as an anticoagulant, an EC 3.4.21.5 (thrombin) inhibitor and an EC 1.10.99.2 [ribosyldihydronicotinamide dehydrogenase (quinone)] inhibitor. It is an aromatic amide, a member of benzimidazoles, a carboxamidine, a member of pyridines and a beta-alanine derivative.
Dabigatran is the active form of the orally bioavailable prodrug [dabigatran etexilate]. Dabigatran is a Direct Thrombin Inhibitor. The mechanism of action of dabigatran is as a Thrombin Inhibitor. Dabigatran is a direct inhibitor of thrombin and anticoagulant which is used for prevention of stroke and venous embolism in patients with chronic atrial fibrillation. Dabigatran therapy has been associated with a low rate of serum enzyme elevations and rare instances of liver enzyme elevations and jaundice. Dabigatran is a benzimidazole and direct thrombin inhibitor, with anticoagulant activity. Upon administration, dabigatran reversibly binds to and inhibits the activity of thrombin, a serine protease that converts fibrinogen into fibrin. This disrupts the coagulation cascade and inhibits the formation of blood clots. A THROMBIN inhibitor which acts by binding and blocking thrombogenic activity and the prevention of thrombus formation. It is used to reduce the risk of stroke and systemic EMBOLISM in patients with nonvalvular atrial fibrillation. See also: Dabigatran Etexilate (is active moiety of); Dabigatran Etexilate Mesylate (active moiety of); Dabigatran Ethyl Ester (is active moiety of). Mechanism of Action Dabigatran and its acyl glucuronides are competitive, direct thrombin inhibitors. Because thrombin (serine protease) enables the conversion of fibrinogen into fibrin during the coagulation cascade, its inhibition prevents the development of a thrombus. Both free and clot-bound thrombin, and thrombin-induced platelet aggregation are inhibited by the active moieties. ... To evaluate the profibrinolytic effect of dabigatran, a new, direct thrombin inhibitor, using different in vitro models. The resistance of tissue factor-induced plasma clots to fibrinolysis by exogenous tissue-type plasminogen activator (t-PA) (turbidimetric method) was reduced by dabigatran in a concentration-dependent manner, with > or = 50% shortening of lysis time at clinically relevant concentrations (1-2 um). A similar effect was observed in the presence of low (0.1 and 1 nm) but not high (10 nm) concentrations of thrombomodulin. Acceleration of clot lysis by dabigatran was associated with a reduction in TAFI activation and thrombin generation, and was largely, although not completely, negated by an inhibitor of activated TAFI, potato tuber carboxypeptidase inhibitor. The assessment of the viscoelastic properties of clots showed that those generated in the presence of dabigatran were more permeable, were less rigid, and consisted of thicker fibers. The impact of these physical changes on fibrinolysis was investigated using a model under flow conditions, which demonstrated that dabigatran made the clots markedly more susceptible to flowing t-PA, by a mechanism that was largely TAFI-independent. Dabigatran, at clinically relevant concentrations, enhances the susceptibility of plasma clots to t-PA-induced lysis by reducing TAFI activation and by altering the clot structure. These mechanisms might contribute to the antithrombotic activity of the drug. Therapeutic Uses Benzimidazoles; beta-Alanine/analogs & derivatives Dabigatran is indicated to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation. /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: PREMATURE DISCONTINUATION OF PRADAXA INCREASES THE RISK OF THROMBOTIC EVENTS. Premature discontinuation of any oral anticoagulant, including Pradaxa, increases the risk of thrombotic events. If anticoagulation with Pradaxa is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant. /BOXED WARNING/ SPINAL/EPIDURAL HEMATOMA. Epidural or spinal hematomas may occur in patients treated with Pradaxa who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include: use of indwelling epidural catheters; concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants; a history of traumatic or repeated epidural or spinal punctures; a history of spinal deformity or spinal surgery; optimal timing between the administration of Pradaxa and neuraxial procedures is not known. Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated. The FDA is evaluating post-marketing reports of serious bleeding events in patients taking dabigatran etexilate mesylate (Pradaxa). Bleeding that may lead to serious or even fatal outcomes is a well-recognized complication of all anticoagulant therapies. The dabigatran drug label contains a warning about significant and sometimes fatal bleeds. In a large clinical trial (18,000 patients) comparing dabigatran and warfarin, major bleeding events occurred at similar rates with the two drugs. FDA is working to determine whether the reports of bleeding in patients taking dabigatran are occurring more commonly than would be expected, based on observations in the large clinical trial that supported the approval of dabigatran. Dabigatran is a blood thinning (anticoagulant) medication used to reduce the risk of stroke in patients with non-valvular atrial fibrillation (AF), the most common type of heart rhythm abnormality. At this time, FDA continues to believe that dabigatran provides an important health benefit when used as directed and recommends that healthcare professionals who prescribe dabigatran follow the recommendations in the approved drug label. Patients with AF should not stop taking dabigatran without talking to their healthcare professional. Stopping use of blood thinning medications can increase their risk of stroke. Strokes can lead to permanent disability and death. Dabigatran is contraindicated in patients with: active pathological bleeding; history of a serious hypersensitivity reaction to dabigatran (e.g., anaphylactic reaction or anaphylactic shock). For more Drug Warnings (Complete) data for Dabigatran (14 total), please visit the HSDB record page. |
| 分子式 |
C25H25N7O3
|
|
|---|---|---|
| 分子量 |
471.51
|
|
| 精确质量 |
471.201
|
|
| 元素分析 |
C, 65.05; H, 6.58; N, 15.62; O, 12.74)
|
|
| CAS号 |
211914-51-1
|
|
| 相关CAS号 |
Dabigatran-d4 hydrochloride;Dabigatran-d3;1246817-44-6;Dabigatran etexilate;211915-06-9;Dabigatran etexilate mesylate;872728-81-9;Dabigatran (ethyl ester);429658-95-7;Dabigatran-d4;1618637-32-3;Dabigatran-13C6;1210608-88-0;Dabigatran-13C,d3
|
|
| PubChem CID |
216210
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
797.1±70.0 °C at 760 mmHg
|
|
| 熔点 |
268-272ºC
|
|
| 闪点 |
435.9±35.7 °C
|
|
| 蒸汽压 |
0.0±2.9 mmHg at 25°C
|
|
| 折射率 |
1.694
|
|
| LogP |
0.79
|
|
| tPSA |
150.22
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
35
|
|
| 分子复杂度/Complexity |
757
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
N=C(N)C(C=C1)=CC=C1NCC2=NC3=CC(C(N(CCC(O)=O)C4=NC=CC=C4)=O)=CC=C3N2C
|
|
| InChi Key |
YBSJFWOBGCMAKL-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C25H25N7O3/c1-31-20-10-7-17(25(35)32(13-11-23(33)34)21-4-2-3-12-28-21)14-19(20)30-22(31)15-29-18-8-5-16(6-9-18)24(26)27/h2-10,12,14,29H,11,13,15H2,1H3,(H3,26,27)(H,33,34)
|
|
| 化学名 |
3-[[2-[(4-carbamimidoylanilino)methyl]-1-methylbenzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1208 mL | 10.6042 mL | 21.2085 mL | |
| 5 mM | 0.4242 mL | 2.1208 mL | 4.2417 mL | |
| 10 mM | 0.2121 mL | 1.0604 mL | 2.1208 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study to Gather Information About the Safety of Oral Anticoagulation Drugs and How Well These Drugs Work in Real World for Patients With Non-valvular Atrial Fibrillation (Irregularly Heart Beats Which is Not Caused by a Heart Valve Problem)
CTID: NCT04249401
Phase: Status: Completed
Date: 2024-08-01