| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
Thrombin
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:达比加群选择性且可逆地抑制人凝血酶(Ki:4.5 nM)以及凝血酶诱导的血小板聚集(IC50:10 nM),同时对其他血小板刺激剂没有抑制作用。达比加群选择性且可逆地抑制人凝血酶 (Ki: 4.5 nM) 以及凝血酶诱导的血小板聚集 (IC50: 10 nM),同时对其他血小板刺激剂没有抑制作用。达比加群抑制贫血小板血浆 (PPP) 中的凝血酶生成,IC50 为 0.56 μM,以内源性凝血酶电位 (ETP) 测量。达比加群在体外对多种物种具有浓度依赖性抗凝作用,在浓度为 0.23、0.83 和 0.18 μM 时,人 PPP 中的活化部分凝血活酶时间 (aPTT)、凝血酶原时间 (PT) 和 ecarin 凝血时间 (ECT) 分别加倍。
|
| 体内研究 (In Vivo) |
大鼠(0.3、1和3 mg/kg)和恒河猴(0.15、0.3和0.6 mg/kg)静脉注射后,达比加群以剂量依赖性方式延长aPTT。与依诺肝素相比,达比加群酯(20 mg/kg,口服)对猪的 K 值延长较小,角度和最大幅度降低较小。达比加群 (0.01-0.1 mg/kg) 减少血栓形成呈剂量依赖性,ED50(有效剂量的 50%)为 0.033 mg/kg,在 0.1 mg/kg 时完全抑制。达比加群酯 (5-30 mg/kg) 以剂量和时间依赖性方式抑制血栓形成,在预处理后 30 分钟内达到最大抑制,表明作用迅速起效。
|
| 动物实验 |
Male rats (280-350 g) and rhesus monkeys of either sex (3-8 kg)
10, 20 and 50 mg/kg for rats and 1, 2.5 and 5 mg/kg for monkeys Oral |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral dabigatran has a bioavailability of 3-7%, although the relative bioavailability of dabigatran pellets is 37% higher than that for capsules and the bioavailability increases to 75% when the capsule shell is removed; dabigatran capsules should not be tampered with in any way prior to administration. The Cmax is achieved by one hour following oral dosing, which is extended to two hours if accompanied by a high-fat meal. Dabigatran can be taken with or without food. Dabigatran pharmacokinetics are approximately linear over a range of 10-400 mg in healthy adults and adult patients and it has an accumulation factor of two in adult and pediatric patients. Dabigatran is primarily eliminated in the urine. Following oral administration of radiolabeled dabigatran, 7% of the radioactivity is recovered in urine and 86% is recovered in feces. Dabigatran has a volume of distribution of 50-70L. Following intravenous administration, renal clearance constitutes ~80% of total dabigatran clearance. Metabolism / Metabolites Dabigatran is administered as the orally available prodrug dabigatran etexilate that is subsequently metabolized to the active form. _In vitro_ studies and observations regarding the oral bioavailability and levels of plasma prodrug suggest extensive first-pass metabolism by carboxylesterases, first by intestinal CES2 to form BIBR0951 (also known as M2) and then subsequently by hepatic CES1 to form [dabigatran]. Dabigatran etexilate can also first undergo CES1-mediated hydrolysis to BIBR1087 (M1) followed by CES2-mediated hydrolysis to [dabigatran], though it is hypothesized that the former pathway accounts for most of the active form in plasma. Dabigatran can undergo 1-_O_-acyl glucuronidation by UGT1A9, UGT2B7, and UGT2B15 followed by acyl migration to form the corresponding 2-_O_-, 3-_O_-, and 4-_O_-acyl glucuronides; all of these acyl glucuronides exhibit activity similar to [dabigatran] but account for a small fraction of recovered metabolites. In addition to these better characterized metabolic pathways, detailed LC/MS characterization suggests a wide variety of possible metabolites following oral or intravenous administration, most of which are present in only trace amounts in plasma, urine, or feces. These include a variety of oxidation, hydrolysis, and conjugation products, including through the addition of mannitol. Biological Half-Life Dabigatran has a half-life of 12-17 hours in adult patients and 12-14 hours in pediatric patients. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Dabigatran is ~35% bound to plasma proteins, including human serum albumin. |
| 参考文献 | |
| 其他信息 |
Dabigatran etexilate is an oral prodrug that is hydrolyzed to the competitive and reversible direct thrombin inhibitor [dabigatran]. Dabigatran etexilate may be used to decrease the risk of venous thromboembolic events in patients in whom anticoagulation therapy is indicated. In contrast to warfarin, because its anticoagulant effects are predictable, lab monitoring is not necessary. Dabigatran etexilate was approved by the FDA in 2010.
A THROMBIN inhibitor which acts by binding and blocking thrombogenic activity and the prevention of thrombus formation. It is used to reduce the risk of stroke and systemic EMBOLISM in patients with nonvalvular atrial fibrillation. See also: Dabigatran (has active moiety); Dabigatran Etexilate Mesylate (has salt form). Drug Indication Dabigatran etexilate is available in both oral pellet and capsule form. Dabigatran etexilate pellets are indicated for the treatment of venous thromboembolic events (VTE) in pediatric patients between three months and 12 years of age who have been treated with a parenteral anticoagulant for at least 5 days. They are also indicated in the same age group to reduce the risk of recurrence of VTE in patients who have been previously treated. In capsule form, dabigatran etexilate is indicated in adults to reduce the risk of stroke and systemic embolism associated with non-valvular atrial fibrillation and for the treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE) in patients who have been treated with a parenteral anticoagulant for 5-10 days. It is also indicated in adults to reduce the risk of recurrence of DVT and PE in patients who have been previously treated and for the prophylaxis of DVT and PE in patients who have undergone hip replacement surgery. Lastly, it is indicated in pediatric patients between eight and 18 years of age for the treatment of venous thromboembolic events (VTE) in patients who have been treated with a parenteral anticoagulant for at least 5 days and to reduce the risk of recurrence of VTE in patients who have been previously treated. Dabigatran etexilate is also approved by the EMA to prevent VTE in adult patients. For pediatric patients, Dabigatran etexilate is used to treat TVE and prevent recurrent TVE for patients from birth to less than 18 years of age. FDA Label Mechanism of Action Hemostasis is a complex process that balances coagulation to prevent excessive thrombus formation or excessive bleeding. Central to the coagulation process is the serine protease thrombin (FIIa), which is synthesized as inactive prothrombin (FII) and subsequently activated by FXa/FVa, leading to a positive feedback loop and the production of large quantities of thrombin; once enough thrombin is formed, it cleaves soluble fibrinogen to form insoluble fibrin fibres that, together with aggregated platelets, form a clot. Although beneficial in wound healing, aberrant thrombus formation can lead to serious health consequences. Dabigatran is a univalent reversible direct thrombin inhibitor (DTI) that competitively inhibits thrombin with a Ki of 4.5 ± 0.2 nmol/L. Furthermore, the reversible nature of the inhibition is believed to allow for some normal physiological thrombin function, which may help alleviate some adverse effects associated with anticoagulation therapy. In addition, dabigatran has several glucuronidated metabolites, all of which have been shown to possess _in vitro_ activity similar to the parent compound. In addition to a direct effect on thrombin activity, dabigatran has also been shown to inhibit platelet aggregation, another step in the coagulation pathway. However, the mechanism remains unclear as dabigatran inhibits platelet aggregation stimulated by thrombin and von Willebrand factor (vWF), but not by other pathways such as ADP- or thromboxane A2-induced aggregation. Pharmacodynamics Dabigatran etexilate is a double prodrug that is hydrolyzed to the active [dabigatran] by intestinal and hepatic carboxylesterases. Dabigatran is a reversible competitive thrombin inhibitor that directly inhibits the conversion by thrombin of fibrinogen to fibrin, impairing the clotting process and acting as an anticoagulant. Dabigatran use prolongs coagulation markers such as the activated partial thromboplastin time (aPTT), ecarin clotting time (ECT), thrombin time (TT), and dilute thrombin time (dTT), but not the international normalized ratio (INR), which cannot be used in this context as it can in [warfarin] monitoring. As with all anticoagulant therapies, dabigatran carries a risk of bleeding, which may increase with concomitant use of antiplatelet agents, fibrinolytic therapy, heparins, or chronic NSAID use, and should be monitored for. Premature discontinuation of dabigatran, in the absence of an alternative anticoagulant, also carries an increased risk of thromboembolic events. Due to the risk of an epidural or spinal hematoma, dabigatran should generally not be used in the context of neuraxial anesthesia or spinal puncture; if such use is unavoidable, careful monitoring should be employed. Dabigatran should not be used in patients with prosthetic heart valves due to an increased occurrence of major bleeding and thromboembolic events. Dabigatran is a substrate of the P-gp transporter and should generally not be administered together with P-gp inhibitors or inducers, especially in patients with impaired renal function. Lastly, dabigatran or any other direct-acting oral anticoagulant should not be administered in patients with triple-positive antiphospholipid syndrome (APS) due to an increased risk of recurrent thrombotic events. In case of the need for emergency reversal, [idarucizumab] is available for use in adult patients; the safety and efficacy of [idarucizumab] has not been established in pediatric patients yet, for whom reversal may be achieved through hemodialysis, prothrombin complex concentrates, or recombinant FVIIa. However, none of these have been sufficiently evaluated in clinical trials. |
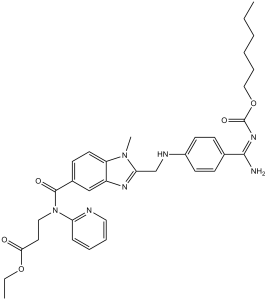
| 分子式 |
C34H41N7O5
|
|
|---|---|---|
| 分子量 |
627.73
|
|
| 精确质量 |
627.316
|
|
| 元素分析 |
C, 65.05; H, 6.58; N, 15.62; O, 12.74)
|
|
| CAS号 |
211915-06-9
|
|
| 相关CAS号 |
Dabigatran;211914-51-1;Dabigatran-d4 hydrochloride;Dabigatran etexilate mesylate;872728-81-9;Dabigatran etexilate-d13;Dabigatran (ethyl ester);429658-95-7;BIBR 1087 SE;212321-78-3
|
|
| PubChem CID |
135565674
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
827.9±75.0 °C at 760 mmHg
|
|
| 熔点 |
128-129°
|
|
| 闪点 |
454.5±37.1 °C
|
|
| 蒸汽压 |
0.0±3.0 mmHg at 25°C
|
|
| 折射率 |
1.615
|
|
| LogP |
5.13
|
|
| tPSA |
154.03
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
9
|
|
| 可旋转键数目(RBC) |
18
|
|
| 重原子数目 |
46
|
|
| 分子复杂度/Complexity |
991
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O(C(/N=C(\C1C([H])=C([H])C(=C([H])C=1[H])N([H])C([H])([H])C1=NC2C([H])=C(C(N(C3=C([H])C([H])=C([H])C([H])=N3)C([H])([H])C([H])([H])C(=O)OC([H])([H])C([H])([H])[H])=O)C([H])=C([H])C=2N1C([H])([H])[H])/N([H])[H])=O)C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H]
|
|
| InChi Key |
XETBXHPXHHOLOE-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C34H41N7O5.CH4O3S/c1-4-6-7-10-21-46-34(44)39-32(35)24-12-15-26(16-13-24)37-23-30-38-27-22-25(14-17-28(27)40(30)3)33(43)41(20-18-31(42)45-5-2)29-11-8-9-19-36-29;1-5(2,3)4/h8-9,11-17,19,22,37H,4-7,10,18,20-21,23H2,1-3H3,(H2,35,39,44);1H3,(H,2,3,4)
|
|
| 化学名 |
ethyl 3-[[2-[[4-(N-hexoxycarbonylcarbamimidoyl)anilino]methyl]-1-methylbenzimidazole-5-carbonyl]-pyridin-2-ylamino]propanoate;methanesulfonic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.98 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.98 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5930 mL | 7.9652 mL | 15.9304 mL | |
| 5 mM | 0.3186 mL | 1.5930 mL | 3.1861 mL | |
| 10 mM | 0.1593 mL | 0.7965 mL | 1.5930 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Learn About How BAY2927088 Affects the Level of Dabigatran or Rosuvastatin in the Blood When These Drugs Are Taken Together in Healthy Participants
CTID: NCT06329895
Phase: Phase 1 Status: Completed
Date: 2024-07-05