| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|

| 靶点 |
Glucocorticoid Receptor
|
|---|---|
| 体外研究 (In Vitro) |
使用甲基强的松龙的主要原因是它可以减轻炎症。常见应用包括短期治疗由各种呼吸系统疾病引起的支气管炎症或急性支气管炎,以及关节炎治疗。甲基泼尼松龙用于治疗自身免疫性疾病,尤其是急性和长期的系统性红斑狼疮。前庭神经炎也可用其治疗[1]。六个月后,运动功能(神经系统变化评分分别为 16.0 和 11.2;P = 0.03)、针刺感觉(变化评分为 11.4 和 6.6;P = 0.02)和触觉(变化评分, 8.9 和 4.3;P = 0.03),与接受安慰剂的患者相比,在受伤后 8 小时内接受甲基强的松龙治疗的患者。无论是在首先被评估为神经系统完全损伤的个体中,还是在被认为具有不完全损伤的个体中[2]。
|
| 体内研究 (In Vivo) |
甲基泼尼松龙可降低电生理学诊断为视神经炎的大鼠 RGC 存活率。甲基强的松龙通过非基因组、钙依赖性机制降低 RGC 存活率。甲基泼尼松龙诱导的 RGC 变性增强取决于钙通过电压门控钙通道的流入。甲基强的松龙治疗导致头端和尾端残端中 ED1 阳性细胞的数量显着减少。损伤后 2、4 和 8 周,甲基强的松龙治疗可显着减少两个脐带残端的组织损失。甲基强的松龙可导致 ED1 阳性细胞和脊髓组织损失的长期减少,减少前庭脊髓纤维的枯死,并在病变后 1 周和 2 周使病变附近的前庭脊髓纤维短暂出芽。剂量为 30 mg/kg 的甲基强的松龙已被证明可有效改善大鼠 SCI 模型的功能结果,抑制 TNF-α 表达和 NF-kB 激活。甲基强的松龙对 NF-kB 功能的抑制可能是通过 IkB 的诱导介导的,IkB 将 NF-kB 捕获在无活性的细胞质复合物中。
|
| 细胞实验 |
人永生化胃上皮GES-1和人单核细胞THP-1在含有10%FBS和1%青霉素-链霉素的RPMI-1640中培养,并在37°C、5%CO2的湿润培养箱中培养。THP-1细胞在RPMI-1640中用100 ng/mL佛波醇12-肉豆蔻酸酯13-乙酸酯(PMA)诱导巨噬细胞(M0)24小时,然后用20 ng/mL IFN-γ和10 ng/mL脂多糖(LPS)进一步孵育24小时以激活巨噬细胞(M1)[3]。
|
| 动物实验 |
Animal/Disease Models: Femoral necrosis mouse model methylprednisolone and lipose-induced head [4]
Doses: 30 mg/kg; 13 mg/kg for 10 days Route of Administration: 30 mg/kg, intramuscularinjection ;Additional oral dose of 13 mg/kg for 10 days resulted in chondrocyte degeneration and fibrocartilage expression after 7 weeks. The density of CD31 and VEGF-R2 markers increased in the femoral head. Adult mice were randomly divided into two groups: experimental and control. Group A (the experimental group) was given (via intramuscular injection) 10 mg/kg of lipopolysaccharide (LPS) and 30 mg/kg of methylprednisolone (MPS). Each mouse additionally received MPS in divided oral doses of 13 mg/kg for 10 consecutive days. Group B (the control group) received normal saline at the same location and same volume as those in Group A. Histological changes of the femoral heads were observed by electron microscopy at 3, 5, and 7 weeks after the last chemical injection. The percentage of empty lacunae was measured randomly and the expression of fibrocartilage was evaluated using an image analyzing system. The expression of CD31 and VEGF-R2 were observed by immunohistochemistry. The bone marrow-derived mononuclear cells were stained with propidium iodide and cell cycle was analyzed by flow cytometry.[4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral methylprednisolone has 89.9% the bioavailability of oral methylprednisolone acetate, while rectal methylprednisolone has 14.2% the bioavailability. Intravitreal methylprednisolone has a Tmax of 2.5h. Approximately 1/10 of an oral or IV dose of methylprednisolone will reach the vitreous humor. Further data regarding the absorption of methylprednisolone are not readily available. Methylprednisolone and its metabolites have been collected in urine in humans. A study in dogs showed 25-31% elimination in urine and 44-52% elimination in feces. The average volume of distribution of methylprednisolone is 1.38L/kg. The average plasma clearance of methylprednisolone is 336mL/h/kg. ORAL ABSORPTION IN SINGLE-DOSE STUDY OF 12 NORMAL MALE VOLUNTEERS. MEAN BIOAVAIL AFTER ORAL ADMIN 89.9%, INDICATING BETTER SYSTEMIC AVAIL OF ESTER THAN ALC. AVG ELIMINATION RATE CONSTANT AFTER ORAL ADMIN OF ESTER & ALC 0.290 H-1, HALF-LIFE OF 2.39 HR. The pharmacokinetics of methylprednisolone (MP) were studied in five normal subjects following intravenous doses of 20, 40 and 80 mg methylprednisolone sodium succinate (MPSS) and an oral dose of 20 mg methylprednisolone as 4 x 5 mg tablets. Plasma concentrations of MP and MPSS were measured by both high performance thin layer (h.p.t.l.c.) and high pressure liquid chromatography (h.p.l.c.). 2. The mean values (+/- s.d.) of half-life, mean residence time (MRT), systemic clearance (CL) and volume of distribution at steady state (Vss) of MP following intravenous administration were 1.93 +/- 0.35 h, 3.50 +/- 1.01 h, 0.45 +/- 0.12 lh-1 kg-1 and 1.5 +/- 0.63 1 kg-1, respectively. There was no evidence of dose-related changes in these values. The plasma MP concentration-time curves were superimposable when normalized for dose. 3. The bioavailability of methylprednisolone from the 20 mg tablet was 0.82 +/- 0.11 (s.d.). 4. In vivo hydrolysis of MPSS was rapid with a half-life of 4.14 +/- 1.62 (s.d.) min, and was independent of dose. In contrast, in vitro hydrolysis in plasma, whole blood and red blood cells was slow; the process continuing for more than 7 days. Sodium fluoride did not prevent the hydrolysis of MPSS. High-dose methylprednisolone is used to treat acute spinal cord injury (ASCI). The objective of the present study was to determine the pharmacokinetics of the pro-drug methylprednisolone hemisuccinate and methylprednisolone in accident victims with ASCI. The patients (n = 26) were treated with a bolus intravenous loading dose of 30 mg/kg MPHS within 2 hr after injury and this was followed by a maintenance infusion of 5.4 mg/kg/h up to 24 hr. Blood, CSF and saliva samples were collected up to 48 hr after the initial dose and the samples were analyzed by HPLC. Concentration-time data of MPHS and methylprednisolone were analyzed using population pharmacokinetic analysis with NONMEM software. RESULTS: Methylprednisolone hemisuccinate and methylprednisolone could be monitored in plasma and CSF. Methylprednisolone but not methylprednisolone hemisuccinate was present in saliva. High variability was seen in the methylprednisolone hemisuccinate levels in CSF. The pharmacokinetics of the pro-drug and the metabolite were adequately described by a 2-compartment model with exponential distribution models assigned to the interindividual and the residual variability. At steady state, the average measured methylprednisolone concentration in plasma was 12.3+/-7.0 microg/ml and 1.74+/-0.85 microg/ml in CSF. The CSF levels of methylprednisolone could be modeled as a part of the peripheral compartment. This study demonstrated that CSF concentrations of methylprednisolone were sufficiently high after IV. administration and reflected the concentrations of unbound drug in plasma. Salivary levels of methylprednisolone were about 32% of the plasma level and may serve as an easily accessible body fluid for drug level monitoring. Sodium fluoride (6--8 mg/ml) inhibits hydrolysis of methylprednisolone acetate to methylprednisolone. An HPLC method for simultaneous determination of hydrocortisone, methylprednisolone and methylprednisolone acetate in plasma is presented. Analysis of plasma samples (containing NaF) for methylprednisolone acetate shows no significant change in concentration over extended periods of storage at -20 degrees C. In vitro hydrolysis of methylprednisolone acetate at 37 degrees C in human whole blood is rapid (average t1/2 = 19 min). In one cat, the bioavailabilities of methylprednisolone acetate rectally was 13% and of methylprednisolone (alcohol) rectally was 26%, relative to intravenous administration of methylprednisolone. In the same cat, the bioavailabilities of methylprednisolone acetate orally was 93% and of methylprednisolone was 82%, relative to intravenous administration of methylprednisolone. All samples collected after oral administration of methylprednisolone acetate to a human subject were found to contain only methylprednisolone (alcohol) indicating hydrolysis of the drug during absorption through the gastrointestinal membrane and/or in the liver. If the ester had the same half-life in blood in vivo as measured in vitro, it would have been measurable in plasma. For more Absorption, Distribution and Excretion (Complete) data for METHYLPREDNISOLONE (7 total), please visit the HSDB record page. Metabolism / Metabolites The metabolism of methylprednisolone is thought to be mostly mediated by 11beta-hydroxysteroid dehydrogenases and 20-ketosteroid reductases. METAB OF 6ALPHA-METHYLPREDNISOLONE NA SUCCINATE IN RATS; METABOLITES; 6ALPHA-METHYLPREDNISOLONE, 6ALPHA-METHYL-11BETA,17ALPHA,20BETA- TRIHYDROXY-1,4-PREGNADIEN-3-ONE,21-OIC ACID, & 6ALPHA-METHYL-11BETA,17ALPHA,20,21-TETRAHYDROXY-1,4-PREGNADIEN-3-ONE 21-SUCCINATE. Prednisone, prednisolone, and methylprednisolone are currently administered in association with cyclosporin A in the postoperative treatment of transplant patients. The aim of this work was to evaluate the effects of these corticosteroids on the expression of several forms of cytochromes p450, including p450 1A2, 2D6, 2E1, and 3A, and on cyclosporin A oxidase activity in human liver. For this purpose, human hepatocytes prepared from lobectomies were maintained in culture in a serum-free medium, in collagen-coated dishes, for 96-144 hr, in the absence or presence of 50-100 uM corticosteroids, rifampicin, or dexamethasone. To mimic more closely the current clinical protocol, hepatocyte cultures were also co-treated with corticosteroids and cyclosporin A or ketoconazole (a selective inhibitor of cytochromes p450 3A). Cyclosporin A oxidase activity, intracellular retention of cyclosporin A oxidized metabolites within hepatocytes, accumulation of cytochromes p450 proteins and corresponding messages, and de novo synthesis and half-lives of these cytochromes p450 were measured in parallel in these cultures. Our results, obtained from seven different hepatocyte cultures, showed that 1) dexamethasone and prednisone, but not prednisolone or methylprednisolone, were inducers of cytochrome p450 3A, at the level of protein and mRNA accumulation, as well as of cyclosporin A oxidase activity, known to be predominantly catalyzed by these cytochromes p450; 2) although corticosteroids are known to be metabolized in human liver, notably by cytochrome p450 3A, partial or total inhibition of this cytochromes p450 by cyclosporin or ketoconazole, respectively, did not affect the inducing efficiency of these molecules; 3) corticosteroids did not affect the half-life of cytochrome p450 3A or the accumulation of other forms of cytochromes p450, including 1A2, 2D6, and 2E1; 4) chronic treatment of cells with cyclosporin did not affect cytochrome p450 3A accumulation; 5) corticosteroids were all competitive inhibitors of cyclosporin A oxidase in human liver microsomes, with Ki values of 61 + or - 12, 125 + or - 25, 190 + or - 38, and 210 + or - 42 uM for dexamethasone, prednisolone, prednisone, and methylprednisolone, respectively; and 6) chronic treatment of cells with corticosteroids did not influence the excretion of oxidized metabolites of cyclosporin from the cells. Biological Half-Life Methylprednisolone has a half life of 2.3h. IV HALF-LIFE IS APPROX 80 MIN IN DOGS. PLASMA HALF-LIFE IS 3-4 HR. SINGLE DOSE STUDY OF 12 NORMAL MALE VOLUNTEERS. HALF-LIFE OF 2.39 HR. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
PHENYTOIN IS ... KNOWN TO INCR METABOLISM OF ... METHYLPREDNISOLONE IN HUMANS. SYSTEMIC EFFECTS OF ADMIN CORTICOSTEROIDS /METHYLPREDNISOLONE/ ... MAY BE DIMINISHED BY LARGE DOSES OF BARBITURATES SUCH AS PHENOBARBITAL. HYPERGLYCEMIC ACTION OF CORTISONE /METHYLPREDNISOLONE/ MAY OFFSET HYPOGLYCEMIC ACTION OF CHLORPROPAMIDE. Methylprednisolone clearance was reduced in asthmatic patients also taking troleandomycin. For more Interactions (Complete) data for METHYLPREDNISOLONE (25 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat subcutaneous > 3,000 mg/kg body weight LD50 Rat (Sprague-Dawley) oral (acute) > 2,000 mg/kg body weight |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Anti-Inflammatory Agents, Steroidal; Antiemetics; Glucocorticoids, Synthetic; Glucocorticoids, Topical; Neuroprotective Agents MEDICATION (VET): Treatment with methylprednisolone may be helpful if instituted within the first few hours of /spinal cord/ injury. MEDICATION (VET): Glucocorticoids are usually contraindicated in animals with meningitis or meningoencephalitis with an infectious etiology; however, a high-dose, short-term course of ... methylprednisolone may control life-threatening complications such as acute cerebral edema and impending brain herniation. MEDICATION (VET): In cats with mild to moderate inflammatory bowel disease (IBD) or relapse of clinical signs, and in those in which administration of oral medication is difficult, methylprednisolone ... may be effective as the sole treatment or as an adjunct to prednisone and metronidazole. For more Therapeutic Uses (Complete) data for METHYLPREDNISOLONE (29 total), please visit the HSDB record page. Drug Warnings /SRP: High dose/ /Methylprednisolone, when administered as a therapeutic agent, has been /associated with hallucinations. /From table/ Contraindicted in patients with systemic fungal infections. Adverse reactions include sodium and fluid retention, potassium depletion, muscle weakness, osteoporosis, peptic ulcer, thin fragile skin, development of Cushingoid state, glaucoma, cataracts, and negative nitrogen balance. May mask signs of infection and new infections may appear during use. May increase requirements for hypoglycemic agents in diabetic patients. We describe a 61 year-old caucasian male diagnosed with rheumatoid arthritis. He was started on methylprednisolone pulses because of a severe flare of symmetric polyarthritis while he was on weekly intramuscular methotrexate and low-dose oral prednisone. After the second pulse of methylprednisolone the patient suddenly developed severe abdominal pain with free air under the right hemidiaphragm in the chest roentgenogram. The emergency surgery revealed the perforation of a colonic diverticulum. We suggest that methylprednisolone pulses should be carefully used in those patients over 50 years of age and/or people with demonstrated or suspected diverticular disease. The immunosuppressive effects of glucocorticoids may result in activation of latent infection or exacerbation of intercurrent infections, including those caused by Candida, Mycobacterium, Toxoplasma, Strongyloides, Pneumocystis, Cryptococcus, Nocardia, or Ameba. Glucocorticoids should be used with great care in patients with known or suspected Strongyloides (threadworm) infection. In such patients, glucocorticoid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia. /Corticosteroids/ For more Drug Warnings (Complete) data for METHYLPREDNISOLONE (33 total), please visit the HSDB record page. Pharmacodynamics Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections. |
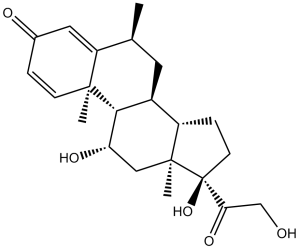
| 分子式 |
C22H30O5
|
|
|---|---|---|
| 分子量 |
374.47
|
|
| 精确质量 |
374.209
|
|
| 元素分析 |
C, 70.56; H, 8.07; O, 21.36
|
|
| CAS号 |
83-43-2
|
|
| 相关CAS号 |
Methylprednisolone acetate;53-36-1;Methylprednisolone (Standard);83-43-2;Methylprednisolone-d7;Methylprednisolone succinate;2921-57-5;Methylprednisolone-d3;Methylprednisolone-d4;Methylprednisolone-d2
|
|
| PubChem CID |
6741
|
|
| 外观&性状 |
Crystals
White to practically white crystalline powder |
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
571.8±50.0 °C at 760 mmHg
|
|
| 熔点 |
228-237°C (dec.)
|
|
| 闪点 |
313.7±26.6 °C
|
|
| 蒸汽压 |
0.0±3.6 mmHg at 25°C
|
|
| 折射率 |
1.603
|
|
| LogP |
1.99
|
|
| tPSA |
94.83
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
754
|
|
| 定义原子立体中心数目 |
8
|
|
| SMILES |
C[C@@]12[C@@](O)(C(=O)CO)CC[C@H]1[C@@H]1C[C@H](C)C3=CC(C=C[C@]3(C)[C@H]1[C@H](C2)O)=O
|
|
| InChi Key |
VHRSUDSXCMQTMA-PJHHCJLFSA-N
|
|
| InChi Code |
InChI=1S/C22H30O5/c1-12-8-14-15-5-7-22(27,18(26)11-23)21(15,3)10-17(25)19(14)20(2)6-4-13(24)9-16(12)20/h4,6,9,12,14-15,17,19,23,25,27H,5,7-8,10-11H2,1-3H3/t12-,14-,15-,17-,19+,20-,21-,22-/m0/s1
|
|
| 化学名 |
(6S,8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-6,10,13-trimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.55 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.55 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.55 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 25 mg/mL (66.76 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液; 超声助溶 (<60°C). *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6704 mL | 13.3522 mL | 26.7044 mL | |
| 5 mM | 0.5341 mL | 2.6704 mL | 5.3409 mL | |
| 10 mM | 0.2670 mL | 1.3352 mL | 2.6704 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。