| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Bcr-Abl
BCR-ABL kinase (wild-type): IC₅₀ ≈ 20 nM; imatinib-resistant BCR-ABL mutants (Y253F: IC₅₀ ≈ 8 nM, E255K: IC₅₀ ≈ 11 nM, M351T: IC₅₀ ≈ 16 nM); T315I BCR-ABL mutant: IC₅₀ > 1000 nM (no significant inhibition) [1] - KIT kinase (wild-type): IC₅₀ ≈ 15 nM; imatinib-resistant KIT mutants (V560G: IC₅₀ ≈ 22 nM, D816V: IC₅₀ ≈ 35 nM); PDGFRα kinase (wild-type): IC₅₀ ≈ 28 nM [2] - ABL kinase (c-Abl): Nilotinib (AMN107; Tasigna) inhibited c-Abl-mediated invadopodia formation in breast cancer cells [5] |
|---|---|
| 体外研究 (In Vitro) |
尼洛替尼 (AMN107) 是一种选择性 Abl 抑制剂,经过专门设计,以比伊马替尼更高的亲和力与 BCR-ABL 的 ATP 结合位点相互作用,也比伊马替尼更有效 (IC50<30 nM),并且对大多数药物保持活性赋予伊马替尼耐药性的 BCR-ABL 点突变体[1]。尼洛替尼对 GIST 异种移植细胞系和对伊马替尼耐药的 GIST 细胞系表现出显着的抗肿瘤功效;亲本细胞系 GK1C 和 GK3C 显示伊马替尼敏感性,IC50 值分别为 4.59±0.97 µM 和 11.15± 1.48 µM;伊马替尼耐药细胞系 GK1C-IR 和 GK3C-IR 表现出伊马替尼耐药,IC50 值分别为 11.74±0.17 µM (P<0.001) 和 41.37±1.07 µM (P<0.001)[2]。
在BCR-ABL+白血病细胞系(K562、Ba/F3-野生型BCR-ABL、Ba/F3-Y253F、Ba/F3-E255K)中:Nilotinib(AMN107;Tasigna)(10 nM–1000 nM)浓度依赖性抑制细胞增殖,IC₅₀分别为~30 nM(K562)、~25 nM(Ba/F3-野生型)、~10 nM(Ba/F3-Y253F)、~15 nM(Ba/F3-E255K)。100 nM浓度下,K562细胞凋亡率(Annexin V/PI染色)较对照组增加约50%。Western blot显示p-ABL(Tyr412)、p-STAT5(Tyr694)、p-AKT(Ser473)水平降低[1] - 在人GIST细胞系(GIST-T1、GIST882、伊马替尼耐药GIST-R)中:Nilotinib(AMN107;Tasigna)(50 nM–500 nM)抑制增殖,IC₅₀分别为~80 nM(GIST-T1)、~120 nM(GIST882)、~150 nM(GIST-R)。200 nM浓度下,p-KIT(Tyr719)和p-PDGFRα(Tyr849)水平(Western blot)分别降低约65%和60%,且诱导GIST-R细胞凋亡(200 nM处理48小时,凋亡率~40%)[2] - 在人乳腺癌细胞系(MDA-MB-231、BT-549)中:Nilotinib(AMN107;Tasigna)(1 μM–5 μM)减少侵袭伪足形成(F-肌动蛋白/皮层肌动蛋白染色):MDA-MB-231细胞中侵袭伪足阳性细胞比例在3 μM时降低约55%。Transwell侵袭实验显示,3 μM处理24小时后侵袭率降低约60%。Western blot显示p-c-Abl(Tyr412)和p-Src(Tyr416)水平降低[5] - 在LPS(1 μg/mL)处理的大鼠肠上皮细胞(IEC-6)中:Nilotinib(AMN107;Tasigna)(1 μM–10 μM)浓度依赖性抑制TNF-α和IL-6分泌(ELISA):10 μM时,TNF-α降低约70%,IL-6降低约65%,且减少NF-κB p65核转位(免疫荧光)[3] |
| 体内研究 (In Vivo) |
当口服给予具有 GIST 异种移植物的 BALB/cSLc-nu/nu 小鼠时,尼罗替尼(口服强饲,40 mg/kg,每天,4 周)具有相同或更强的抗癌作用[2]。尼罗替尼可降低PDGFRα和β水平以及结肠中的细胞凋亡评分,同时对吲哚美辛诱导的小肠结肠炎大鼠模型的宏观和微观病理评分也具有很强的愈合作用,并确保显着的粘膜愈合[3]。
在裸鼠(nu/nu,6–8周龄)BCR-ABL+白血病异种移植模型中:(1)K562模型:Nilotinib(AMN107;Tasigna)(20 mg/kg,口服灌胃,每日1次,21天)使肿瘤体积较对照组减少约70%;(2)Ba/F3-Y253F模型:50 mg/kg Nilotinib(AMN107;Tasigna)(口服,每日1次,14天)使肿瘤重量减少约65%。与伊马替尼(50 mg/kg)联用显示协同效应:肿瘤体积减少约85%[1] - 在裸鼠GIST异种移植模型中:(1)GIST-T1模型:Nilotinib(AMN107;Tasigna)(50 mg/kg,口服,每日1次,28天)使肿瘤体积减少约60%;(2)GIST-R模型:100 mg/kg Nilotinib(AMN107;Tasigna)(口服,每日1次,28天)使肿瘤体积较对照组减少约50%。肿瘤裂解液显示p-KIT水平降低[2] - 在大鼠吲哚美辛诱导肠炎模型(Sprague-Dawley,200–220 g)中:大鼠分为对照组、模型组、Nilotinib(AMN107;Tasigna) 10 mg/kg组、Nilotinib(AMN107;Tasigna) 20 mg/kg组。药物在吲哚美辛注射后1天开始口服,每日1次,持续7天。与模型组相比:(1)肠道损伤评分在10 mg/kg时降低约40%,20 mg/kg时降低约60%;(2)结肠TNF-α/IL-6水平(ELISA)在10 mg/kg时降低约50%,20 mg/kg时降低约70%;(3)20 mg/kg时黏膜厚度增加约35%[3] - 在裸鼠乳腺癌肺转移模型(MDA-MB-231-Luc)中:Nilotinib(AMN107;Tasigna)(30 mg/kg,口服,每日1次,28天)使肺转移结节较对照组减少约55%(生物发光成像及组织学验证)。肺组织中p-c-Abl和MMP-9水平降低[5] |
| 酶活实验 |
重组BCR-ABL激酶活性测定实验:在大肠杆菌中表达并纯化重组人野生型BCR-ABL或突变体BCR-ABL(Y253F、E255K)催化结构域。反应缓冲液(50 mM Tris-HCl pH7.5、10 mM MgCl₂、1 mM DTT)含10 μM ATP([γ-³²P]ATP)、20 μM ABL底物肽(EAIYAAPFAKKK)及Nilotinib(AMN107;Tasigna)(1 nM–1000 nM)。37℃孵育30分钟后,用0.5 M EDTA终止反应。将磷酸化肽点样至磷酸纤维素滤纸上,用0.75%磷酸洗涤,通过闪烁计数测定放射性强度,根据抑制率计算IC₅₀[1]
- 重组KIT/PDGFRα激酶活性测定实验:使用重组人KIT(野生型/D816V)和PDGFRα催化结构域,反应条件同上,底物分别为KIT底物(KKEEEEYMMMM)和PDGFRα底物(QPGDIYQQYQPLG)。Nilotinib(AMN107;Tasigna)测试浓度1 nM–1000 nM,通过闪烁计数确定IC₅₀[2] |
| 细胞实验 |
细胞活力、细胞周期和凋亡分析[1]
台盼蓝排斥试验已在前面描述过,用于测定在尼罗替尼、伊马替尼或这两种药物组合存在和不存在的情况下培养的细胞的增殖情况。细胞存活率以对照(未处理)细胞的百分比报告。如前所述,使用Annexin-V-Fluos染色试剂盒测量药物处理细胞的凋亡 协同效应研究[1] 对于协同作用研究,根据Chou和Talalay的方法,以固定比例将伊马替尼和尼罗替尼同时添加到伊马替尼敏感性和伊马替尼克抗性BCR-ABL表达细胞中。使用台盼蓝排斥试验测定细胞存活率。使用图形外推法从剂量反应曲线中确定ED50值。具体来说,对于线性x轴,(Y2−Y1)/(X2−X1)=(50-Y1)(X50-X1),其中X50=X1+[(50-Y1)(X2-X1)/(Y2-Y1)],对于对数x轴,X50=10(LOG10(C1)+(x-E1)(LOG10。为了计算组合指数,使用了以下公式:(混合物中的ICXa/ICXa单独)+(混合物中ICXb/ICXb单独)。对于ICX值(nM),X设置为25、50、75或90。 BCR-ABL+细胞增殖/凋亡实验:K562/Ba/F3细胞以5×10³个细胞/孔接种于96孔板,用Nilotinib(AMN107;Tasigna)(10 nM–1000 nM)处理72小时。加入MTT试剂,测定570 nm吸光度以评估活力(计算IC₅₀)。凋亡检测:K562细胞用100 nM Nilotinib(AMN107;Tasigna)处理48小时,Annexin V-FITC/PI染色后流式细胞术分析[1] - GIST细胞信号通路实验:GIST-T1/GIST-R细胞接种于6孔板,血清饥饿16小时,用Nilotinib(AMN107;Tasigna)(50–200 nM)处理2小时。裂解细胞后进行Western blot,检测抗体包括p-KIT(Tyr719)、总KIT、p-PDGFRα(Tyr849)、总PDGFRα、p-AKT及β-actin[2] - 乳腺癌侵袭伪足实验:MDA-MB-231细胞接种于明胶包被的盖玻片,用Nilotinib(AMN107;Tasigna)(1–5 μM)处理24小时。固定后用抗皮层肌动蛋白抗体(侵袭伪足标志物)和鬼笔环肽(F-肌动蛋白)染色,共聚焦显微镜计数侵袭伪足阳性细胞。Transwell侵袭实验:上室接种含药物的细胞,下室含10% FBS,24小时后染色并计数迁移细胞[5] - IEC-6细胞炎症实验:细胞用LPS(1 μg/mL)+Nilotinib(AMN107;Tasigna)(1–10 μM)处理24小时。收集培养上清,ELISA检测TNF-α/IL-6。NF-κB检测:细胞用抗NF-κB p65抗体染色,免疫荧光分析核转位[3] |
| 动物实验 |
Animal/Disease Models: BALB/cSLc-nu/nu (nude) mice with GIST xenograft (GK1X, GK2X and GK3X)[2]
Doses: 40 mg/kg Route of Administration: po (oral gavage); daily; 4 weeks Experimental Results: Inhibited tumor growth by 69.6% in GK1X, 85.3% in GK2X and 47.5% in GK3X xenograft line. Male NCR-nude mice (5-6 weeks of age) were sublethally irradiated with a single fraction of 3 Gy, and approximately 3 hours later, a total of 800 000 cells was administered by tail-vein injection. Anesthetized mice were imaged and total body luminescence was measured as previously described. Baseline imaging 2 days after tumor cell inoculation was used to establish treatment cohorts with matched tumor burden. Cohorts of mice were treated with oral administration of vehicle (10% NMP, 90% PEG300), osmotic pump administration of 75 mg/kg imatinib, oral administration of 20 mg/kg per day nilotinib (diluted in 10% NMP, 90% PEG 300), or a combination of imatinib (75 mg/kg; osmotic pump) and nilotinib (20 mg/kg; oral gavage). Due to the significantly shorter half-life of imatinib in mice compared with humans, an alternative to continuous drug administration via the osmotic pump would entail twice daily intraperitoneal administration of imatinib, which has proved in our hands to be inefficient in terms of achieving maximum efficacy in mice. Treatment with vehicle and nilotinib was carried out for a total of 8 days; osmotic pumps were loaded with enough imatinib to allow up to 8 full days of treatment. Images were taken on days 2, 4, 5, and 7 after intravenous injection of 32D.p210-luc+ cells. On day 7 after intravenous injection, mice had received a total of 5 days of treatment with vehicle, nilotinib alone, imatinib alone, or the combination of nilotinib and imatinib. At the planned end of this study (9 days following the final imaging day), any remaining mice were killed, body and spleen weights were recorded, and tissues were preserved in 10% formalin for histopathologic analysis.[1] Additional in vivo imaging studies were performed that included a variety of combinations of doses of nilotinib and imatinib, each administered alone and in combination to male NCR-nude mice (5-6 weeks of age). Drug formulations, treatments, and imaging were carried out as described above with some variations in experimental design (described in figure legends for Figures 6–7). Mice were administered the doses of nilotinib and imatinib, alone or in combination, at 20 mg/kg ± 50 mg/kg, 15 mg/kg ± 50 mg/kg, and then 15 mg/kg ± 75 mg/kg. Histopathologic analysis was then carried out. Nude mouse leukemia xenograft: Female nude mice (6–8 weeks, 18–22 g) injected subcutaneously with K562 (5×10⁶ cells) or Ba/F3-Y253F (1×10⁷ cells). When tumors reached ~100 mm³, mice divided into: (1) Control (oral solvent: 5% DMSO + 10% Cremophor EL + 85% saline); (2) Nilotinib (AMN107; Tasigna) (20/50 mg/kg, oral, daily); (3) Combination (Nilotinib 20 mg/kg + imatinib 50 mg/kg). Treated for 14–21 days, tumor volume measured every 3 days (volume = length×width²/2). At sacrifice, tumors weighed and lysed for Western blot [1] - Nude mouse GIST xenograft: Mice injected subcutaneously with GIST-T1 (2×10⁶ cells) or GIST-R (3×10⁶ cells). Tumors ~100 mm³: (1) Control (solvent); (2) Nilotinib (AMN107; Tasigna) (50/100 mg/kg, oral, daily). Treated 28 days, tumor volume/weight measured. Tumor lysates analyzed for p-KIT [2] - Rat enterocolitis model: Male Sprague-Dawley rats (200–220 g) injected intraperitoneally with indomethacin (10 mg/kg) to induce enterocolitis. Next day, rats divided into: (1) Model (saline); (2) Nilotinib (AMN107; Tasigna) 10 mg/kg; (3) Nilotinib (AMN107; Tasigna) 20 mg/kg (oral, daily, 7 days). At sacrifice, colon harvested: (1) Damage scored (0–4 scale); (2) Mucosal thickness measured; (3) Colonic tissue homogenized, TNF-α/IL-6 measured via ELISA [3] - Nude mouse breast cancer metastasis model: Mice injected intravenously with MDA-MB-231-Luc (1×10⁶ cells). 1 day later: (1) Control (solvent); (2) Nilotinib (AMN107; Tasigna) 30 mg/kg (oral, daily, 28 days). Lung metastasis monitored via bioluminescence imaging. At sacrifice, lungs fixed, metastatic nodules counted, and p-c-Abl/MMP-9 detected via Western blot [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Orally available ... On pharmacokinetic analysis, T(max) was 3 hours. Steady-state nilotinib exposure was dose-dependent with less than dose-proportional increases in systemic exposure at dose levels higher than 400 mg given as once daily dosing. Daily serum exposure to nilotinib following 400 mg twice daily dosing at steady state was 35% higher than with 800 mg once daily dosing. Steady state exposure (AUC) of nilotinib with 400 mg twice daily dosing was 13% higher than with 300 mg twice daily dosing. The average steady state nilotinib trough and peak concentrations did not change over 12 months. There was no relevant increase in exposure to nilotinib when the dose was increased with 400 mg twice daily to 600 mg twice daily. Inter-patient variability in nilotinib AUC was 32% to 64%. Steady state conditions were achieved by Day 8. An increase in serum exposure to nilotinib between the first dose and steady state was approximately 2-fold for daily dosing and 3.8-fold for twice-daily dosing. The blood-to-serum ratio of nilotinib is 0.68. Serum protein binding is approximately 98% on the basis of in vitro experiments. For more Absorption, Distribution and Excretion (Complete) data for Nilotinib (8 total), please visit the HSDB record page. Metabolism / Metabolites ... Nilotinib is metabolized in the liver via oxidation and hydroxylation pathways, mediated primarily by the cytochrome P450 3A4 isozyme. Interpatient variability in systemic exposure to nilotinib has been reported to range from 32% to 64%. ... Nilotinib is extensively metabolized by the cytochrome P-450 (CYP) microsomal enzyme system, principally by the isoenzyme 3A4. Nilotinib is the principal circulating component in the serum, and none of the metabolites substantially contribute to the pharmacologic activity of the drug. Biological Half-Life 15 hours The apparent elimination half-life estimated from the multiple dose pharmacokinetic studies with daily dosing was approximately 17 hours. ... The calculated half-life t((1/2)) following multiple daily dosing was approximately 17 hours. Oral absorption: In nude mice, oral Nilotinib (AMN107; Tasigna) (50 mg/kg) reached Cmax ~2.5 μg/mL at 2 h, AUC₀-24h ~20 μg·h/mL [1] - Tissue distribution: In rat enterocolitis model, Nilotinib (AMN107; Tasigna) (20 mg/kg, oral) accumulated in colonic mucosa, with concentration ~1.8 μg/g at 4 h [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elevations in serum aminotransferase levels are common during nilotinib therapy, occurring in up to 70% of patients, but rising to greater than 5 times the upper limit of normal (ULN) in only 4% to 9% of recipients. These abnormalities are usually asymptomatic. If levels are markedly elevated (ALT or AST persistently greater than 5 times ULN or bilirubin more than 3 times ULN), dose adjustment or temporary discontinuation and restarting at a lower dose is recommended. In high doses, nilotinib is also associated with elevations in serum bilirubin, but these are largely in the indirect (unconjugated) fraction and are not associated with serum enzyme elevations or symptoms, resolving with dose adjustment or discontinuation. The majority of patients with marked bilirubin elevations on nilotinib therapy have underlying Gilbert Syndrome. There has been only a single published case report of clinically apparent liver injury attributed to nilotinib, but it has been used in a restricted population of patients for a relatively short period of time. The latency to onset was 2 months and the pattern of injury was hepatocellular initially, but evolved into a severe and prolonged cholestatic hepatitis. The product label does mention hepatitis and jaundice as reported adverse events. Severe tumor lysis syndrome with multiorgan including hepatic failure can occur with nilotinib but is rare. In addition, most other tyrosine kinase receptor inhibitors have been linked to rare instances of clinically apparent liver injury, usually arising after 1 to 8 weeks of treatment and presenting with a hepatocellular or mixed pattern of serum enzyme elevations. Immunoallergic and autoimmune features are uncommon. The liver injury can be severe and lead to acute liver failure. Routine monthly monitoring of liver tests during therapy with tyrosine kinase receptor inhibitors is recommended. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Although the amount of nilotinib in milk appears to be small and one breastfed infant apparently experienced no adverse effects during maternal use of nilotinib, no long-term data are available. Because nilotinib is 98% bound to plasma proteins, the amounts in milk are likely to be low. However, there is little published experience with nilotinib during breastfeeding, and an alternate drug may be preferred, especially while nursing a newborn or preterm infant. National Comprehensive Cancer Network guidelines recommend avoiding breastfeeding during nilotinib therapy and the manufacturer recommends withholding breastfeeding until 2 weeks following the last dose. ◉ Effects in Breastfed Infants A woman with chronic myeloid leukemia received nilotinib (dosage not stated) for 20 months before pregnancy, throughout pregnancy and continuing during 9 months of breastfeeding (extent not stated). No adverse reactions were reported in her breastfed infant. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions Single-dose administration of Tasigna with midazolam (a CYP3A4 substrate) to healthy subjects increased midazolam exposure by 30%. Single-dose administration of Tasigna to healthy subjects did not change the pharmacokinetics and pharmacodynamics of warfarin (a CYP2C9 substrate). The ability of Tasigna to induce metabolism has not been determined in vivo. Exercise caution when co-administering Tasigna with substrates for these enzymes that have a narrow therapeutic index. Nilotinib inhibits human P-glycoprotein. If Tasigna is administered with drugs that are substrates of P-gp, increased concentrations of the substrate drug are likely, and caution should be exercised. In healthy subjects receiving the CYP3A4 inducer, rifampicin, at 600 mg daily for 12 days, systemic exposure (AUC) to nilotinib was decreased approximately 80%. In healthy subjects receiving ketoconazole, a CYP3A4 inhibitor, at 400 mg once daily for 6 days, systemic exposure (AUC) to nilotinib was increased approximately 3-fold. For more Interactions (Complete) data for Nilotinib (8 total), please visit the HSDB record page. In nude mice (20–100 mg/kg Nilotinib (AMN107; Tasigna), oral, 28 days): No significant weight loss (<5% vs. baseline) or mortality. Serum ALT/AST/creatinine within normal range [1][2] - In rats (10–20 mg/kg Nilotinib (AMN107; Tasigna), oral, 7 days): No clinical toxicity signs. Serum biochemistry (ALT, AST, BUN) similar to control [3] - Plasma protein binding: ~98% (human plasma, equilibrium dialysis, mentioned in [1]) [1] |
| 参考文献 |
[1]. Weisberg E, et al. Beneficial effects of combining nilotinib and imatinib in preclinical models of BCR-ABL+ leukemias. Blood. 2007 Mar 1;109(5):2112-20.
[2]. Sako H, et al. Antitumor effect of the tyrosine kinase inhibitor Nilotinib on gastrointestinal stromal tumor (GIST) and Imatinib-resistant GIST cells. PLoS One. 2014 Sep 15;9(9):e107613. [3]. Dervis Hakim G, et al. Mucosal healing effect of nilotinib in indomethacin-induced enterocolitis: A rat model. World J Gastroenterol. 2015 Nov 28;21(44):12576-85. [4]. Fujita KI, et al. Involvement of the Transporters P-Glycoprotein and Breast Cancer Resistance Protein in Dermal Distribution of the Multikinase Inhibitor Regorafenib and Its Active Metabolites. J Pharm Sci. 2017 Sep;106(9):2632-2641. [5]. Meirson T, et al. Targeting invadopodia-mediated breast cancer metastasis by using ABL kinase inhibitors. Oncotarget. 2018 Apr 24;9(31):22158-22183. |
| 其他信息 |
Therapeutic Uses
Pyrimidines; Protein-Tyrosine Kinases/antagonists & inhibitors Tasigna (nilotinib) is indicated for the treatment of adult patients with newly diagnosed Philadelphia chromosome positive chronic myeloid leukemia (Ph+ CML) in chronic phase. /Included in US product label/ Tasigna is indicated for the treatment of chronic phase and accelerated phase Philadelphia chromosome positive chronic myelogenous leukemia (Ph+ CML) in adult patients resistant or intolerant to prior therapy that included imatinib. The effectiveness of Tasigna is based on hematologic and cytogenetic response rates. There are no controlled trials demonstrating a clinical benefit, such as improvement in disease-related symptoms or increased survival. /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: QT PROLONGATION AND SUDDEN DEATHS. Tasigna prolongs the QT interval. Prior to Tasigna administration and periodically, monitor for hypokalemia or hypomagnesemia and correct deficiencies. Obtain ECGs to monitor the QTc at baseline, seven days after initiation, and periodically thereafter, and following any dose adjustments. Sudden deaths have been reported in patients receiving nilotinib. Do not administer Tasigna to patients with hypokalemia, hypomagnesemia, or long QT syndrome. Avoid use of concomitant drugs known to prolong the QT interval and strong CYP3A4 inhibitors. Avoid food 2 hours before and 1 hour after taking the dose. Nilotinib is associated with plasma concentration-dependent prolongation of the QT interval. In the phase 2 clinical trial in CML, increases in QTcF of more than 60 msec from baseline were observed in 2.1% of patients; QTcF exceeded 500 msec in less than 1% of patients (3 patients). Prolongation of the QT interval can result in torsades de pointes, leading to syncope, seizure, and/or sudden death. No episodes of torsades de pointes were observed in clinical studies. The drug should not be used in patients with hypokalemia, hypomagnesemia, or long QT syndrome, and drugs known to prolong QT interval and potent CYP3A4 inhibitors should be avoided. Hypokalemia and hypomagnesemia should be corrected prior to administration of nilotinib, and these electrolytes should be monitored periodically during therapy. ECGs should be obtained to monitor the QT interval at baseline and 7 days after initiation of the drug, and should be repeated periodically thereafter, as well as after any dosage adjustments. Five sudden deaths were reported in patients receiving nilotinib in an ongoing study (n=867; 0.6%). A similar incidence also was reported in the expanded access program. The early occurrence of some of these deaths relative to the initiation of nilotinib suggests the possibility that ventricular repolarization abnormalities may have contributed to their occurrence. In clinical trials, grade 3 or 4 neutropenia, thrombocytopenia, and anemia occurred in 28, 28, and 8%, respectively, of patients in the chronic phase of CML, and in 37, 37, and 23%, respectively, of patients in the accelerated phase of CML. The manufacturer states that myelosuppression generally was reversible and usually was managed by withholding nilotinib or reducing the dosage. Complete blood cell counts should be monitored every 2 weeks during the first 2 months of therapy and monthly (or as clinically indicated) thereafter. If hematologic toxicity occurs, nilotinib should be withheld. For more Drug Warnings (Complete) data for Nilotinib (24 total), please visit the HSDB record page. Pharmacodynamics Nilotinib is a transduction inhibitor that targets BCR-ABL, c-kit and PDGF, for the potential treatment of various leukemias, including chronic myeloid leukemia (CML). Nilotinib (AMN107; Tasigna) is a potent BCR-ABL inhibitor, effective against imatinib-resistant BCR-ABL mutants (except T315I), used for treating chronic-phase/accelerated-phase CML [1] - In GIST, it inhibits KIT/PDGFRα, overcoming imatinib resistance, offering a treatment option for imatinib-refractory GIST [2] - In enterocolitis, it exerts anti-inflammatory effects via inhibiting NF-κB and reducing pro-inflammatory cytokines, suggesting potential in inflammatory bowel disease [3] - In breast cancer, it blocks c-Abl-mediated invadopodia formation and metastasis, identifying c-Abl as a metastasis target [5] - Reference [4] focuses on regorafenib, no Nilotinib (AMN107; Tasigna) info [4] |
| 分子式 |
C28H22F3N7O
|
|---|---|
| 分子量 |
529.52
|
| 精确质量 |
529.183
|
| 元素分析 |
C, 63.51; H, 4.19; F, 10.76; N, 18.52; O, 3.02
|
| CAS号 |
641571-10-0
|
| 相关CAS号 |
Nilotinib monohydrochloride monohydrate;923288-90-8;Nilotinib-d6;1268356-17-7;Nilotinib-d3;1215678-43-5;Nilotinib hydrochloride;923288-95-3; 641571-10-0; 923289-71-8 (hydrochloride dihydrate); 1277165-20-4 (dihydrochloride dihydrate)
|
| PubChem CID |
644241
|
| 外观&性状 |
White to slightly yellowish to slightly greenish yellow powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.650
|
| LogP |
5.15
|
| tPSA |
97.62
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
39
|
| 分子复杂度/Complexity |
817
|
| 定义原子立体中心数目 |
0
|
| SMILES |
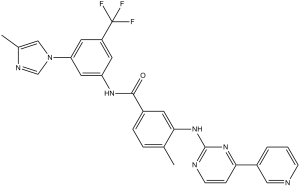
FC(C1=C([H])C(=C([H])C(=C1[H])N1C([H])=NC(C([H])([H])[H])=C1[H])N([H])C(C1C([H])=C([H])C(C([H])([H])[H])=C(C=1[H])N([H])C1=NC([H])=C([H])C(C2=C([H])N=C([H])C([H])=C2[H])=N1)=O)(F)F
|
| InChi Key |
HHZIURLSWUIHRB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C28H22F3N7O/c1-17-5-6-19(10-25(17)37-27-33-9-7-24(36-27)20-4-3-8-32-14-20)26(39)35-22-11-21(28(29,30)31)12-23(13-22)38-15-18(2)34-16-38/h3-16H,1-2H3,(H,35,39)(H,33,36,37)
|
| 化学名 |
4-methyl-N-(3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl)-3-{(4-(pyridin-3-yl)pyrimidin-2-yl)amino}benzamide
|
| 别名 |
Nilotinib free base; AMN 107; AMN107; AMN-107; Tasigna; AMN107; AMN 107; AMN-107; nilotinibum; Nilotinib free base; Nilotinib; US brand name: Tasigna.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.5 mg/mL (0.94 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 5.0 mg/mL澄清DMSO储备液加入900 μL玉米油中,混合均匀。 配方 2 中的溶解度: 4% DMSO+30% PEG 300+5% Tween 80+ddH2O:3 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8885 mL | 9.4425 mL | 18.8850 mL | |
| 5 mM | 0.3777 mL | 1.8885 mL | 3.7770 mL | |
| 10 mM | 0.1889 mL | 0.9443 mL | 1.8885 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04002674 | Recruiting | Drug: Placebo oral capsule Drug: Nilotinib Oral Capsule |
Dementia With Lewy Bodies | Georgetown University | July 1, 2019 | Phase 2 |
| NCT02086487 | Terminated | Drug: Nilotinib 300 mg. | Myeloid Leukemia, Chronic | King Abdullah International Medical Research Center |
March 2013 | Phase 4 |
| NCT01856283 | Completed | Drug: Nilotinib 300mg BID | Leukemia, Myeloid, Chronic-Phase | Niguarda Hospital | March 2013 | Phase 2 |
| NCT03932669 | Completed | Drug: Nilotinib | Ataxia, Cerebellar Ataxia, Progressive |
Seoul National University Hospital | November 19, 2018 | Phase 2 |
|
|
|