| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g | |||
| 10g | |||
| Other Sizes |

| 靶点 |
DPP-4 (IC50 = 18 nM)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:作为口服活性剂,西他列汀磷酸盐对 DPP-4 表现出有效的抑制作用,Caco-2 细胞提取物的 IC50 为 19 nM。 MK0431 通过涉及 cAMP/PKA/Rac1 激活的途径减少分离的脾 CD4 T 细胞的体外迁移。最近的一项研究表明,西他列汀发挥一种新颖的直接作用,通过不依赖 DPP-4、依赖蛋白激酶 A 和 MEK-ERK1/2 的途径刺激肠道 L 细胞分泌 GLP-1。因此,它减少了自身免疫对移植物存活的影响。激酶测定:DPP-4 是从汇合的 Caco-2 细胞中提取的。使用裂解缓冲液(10 mM Tris-HCl、150 mM NaCl、0.04 U/mL 抑肽酶、0.5% Nonidet P40、pH 8.0)在室温下孵育 5 分钟后,将细胞在 4 °C 下以 35,000 g 离心 30 分钟,上清液保存于-80°C。通过将 20 μL 适当的化合物稀释液与 50 μL DPP-4 酶的底物 H-Ala-Pro-7-amido-4-三氟甲基香豆素(测定中的最终浓度,100 μM)和 30 μL 混合来进行测定Caco-2 细胞提取物(用 100 mM Tris-HCl、100 mM NaCl、pH 7.8 稀释 1000 倍)。将板在室温下孵育 1 小时,并使用 SpectraMax GeminiXS 在 405/535 nm 的激发/发射波长下测量荧光。将 Caco-2 细胞提取物与高抑制剂浓度(BI 1356 为 30 nM,维格列汀为 3 μM)预孵育 1 小时后,测定抑制剂与 DPP-4 酶的解离动力学。用测定缓冲液将预孵育混合物稀释 3000 倍后,添加底物 H-Ala-Pro-7-amido-4-triflumethylcoumarin,开始酶促反应。在这些条件下,在存在或不存在抑制剂的情况下,在某个时间点DPP-4活性的差异反映了仍然与DPP-4酶结合的该抑制剂的量。使用 SpectraMax 的 SoftMax 软件计算 10 分钟间隔的最大反应速率(荧光单位/秒 × 1000),并针对未抑制反应的速率进行校正 [(vcontrol-vinhibitor)/vcontrol]。细胞测定:将 CD4T 细胞铺在无血清 RPMI 1640 的膜插入物上,并在存在或不存在纯化猪肾 DPP-4(32.1 单位/mg;100 mU)的情况下使用 Transwell 小室(Corning)测定细胞迁移/mL 最终浓度)和 DPP-4 抑制剂(100 μM)。 1小时后,机械除去上表面的细胞,对迁移到下室的细胞进行计数。迁移程度是相对于对照样品来表示的。
|
| 体内研究 (In Vivo) |
在体内,在自由喂养的 Han-Wistar 大鼠中,磷酸西格列汀抑制血浆 DPP-4 活性的 ED50 值计算为给药后 7 小时 2.3 mg/kg 和给药后 24 小时 30 mg/kg。链脲佐菌素诱导的 1 型糖尿病小鼠模型表现出血浆中 DPP-4 水平升高,而在服用磷酸西他列汀饮食的小鼠中,DPP-4 水平可得到显着抑制。这是通过对高血糖调节的积极作用来实现的,可能是通过延长胰岛移植物的存活时间来实现的。大鼠中磷酸西格列汀的血浆清除率和分布容积(40-48 mL/min/kg,7-9 L/kg)高于狗(9 mL/min/kg,3 L/kg);其半衰期在大鼠中较短,为2小时,而在狗中为4小时。
|
| 酶活实验 |
汇合的 Caco-2 细胞用于提取 DPP-4。用裂解缓冲液(10 mM Tris-HCl、150 mM NaCl、0.04 U/mL 抑肽酶、0.5% Nonidet P40、pH 8.0)在室温下孵育 5 分钟后,将细胞在 35,000 g、4 ℃下离心 30 分钟。 °C,然后将上清液保存在-80°C。将二十微升合适的化合物稀释液与五十微升作为 DPP-4 酶底物的 H-Ala-Pro-7-酰胺基-4-三氟甲基香豆素(测定中的最终浓度:100 微升)和三十微升Caco-2 细胞提取物(用 100 mM Tris-HCl、100 mM NaCl、pH 7.8 稀释 1000 倍)。将板在室温下孵育一小时后,使用 SpectraMax GeminiXS 在 405/535 nm 的激发/发射波长下测量荧光。将 Caco-2 细胞提取物暴露于高浓度抑制剂(BI 1356 为 30 nM,维格列汀为 3 μM)一小时后,确定抑制剂与 DPP-4 酶的解离动力学。一旦用测定缓冲液将预孵育混合物稀释 3000 倍,就通过添加底物 H-Ala-Pro-7-amido-4-triflumethylcoumarini 来启动酶促反应。仍然与DPP-4酶结合的抑制剂的量通过在存在或不存在抑制剂的情况下给定时间的DPP-4活性的差异来指示。使用 SpectraMax 的 SoftMax 软件,以 10 分钟的间隔计算最大反应速率(荧光单位/秒 × 1000),并针对未抑制反应的速率进行校正 [(vcontrol-vinhibitor)/vcontrol]。
|
| 细胞实验 |
将含有 CD4T 细胞的膜插入物铺板于无血清 RPMI 1640 中。使用 Corning Transwell 小室测量细胞迁移,使用或不使用 DPP-4 抑制剂 (100 μM) 和纯化的猪肾 DPP-4(32.1 单位/毫克;最终浓度为 100 mU/mL)。一小时后,对移入下室的细胞进行计数,并机械去除上表面的细胞。迁移量的表达式与对照样品相关。
胰高血糖素样肽-1(GLP-1)是由肠L细胞分泌到循环中的肠促胰岛素激素。二肽基肽酶IV(DPP-IV)抑制剂西格列汀可防止GLP-1降解,并在临床上用于治疗2型糖尿病患者,从而改善糖化血红蛋白水平。当在2型糖尿病模型新生链脲佐菌素大鼠中检查西格列汀对GLP-1水平的影响时,观察到活性GLP-1的基础血浆水平增加4.9±0.9倍,口服葡萄糖刺激的血浆水平增加3.6±0.4倍(P<0.001),肠道L细胞总数增加1.5±0.1倍(P<0.01)。因此,在体外小鼠GLUTag(mGLUTag)和人hNCI-H716肠L细胞中研究了西格列汀对GLP-1分泌和L细胞信号传导的直接影响。西格列汀(0.1-2μM)增加了mGLUTag和hNCI-H716细胞的GLP-1总分泌量(P<0.01-0.001)。然而,MK0626(1-50μM)是一种结构上无关的DPP-IV抑制剂,在两种模型中均不影响GLP-1的分泌。用GLP-1受体激动剂毒蜥外泌肽-4处理mGLUTag细胞没有调节GLP-1的释放,表明GLP-1对L细胞没有反馈作用。西格列汀增加了mGLUTag和hNCI-H716细胞中的cAMP水平(P<0.01)和ERK1/2磷酸化(P<0.05),但没有改变细胞内钙或磷酸化Akt水平。用蛋白激酶A(H89和蛋白激酶抑制剂)或MAPK激酶-ERK1/2(PD98059和U0126)抑制剂预处理mGLUTag细胞可防止西格列汀诱导的GLP-1分泌(P<0.05-0.01)。这些研究首次证明,西格列汀对肠道L细胞具有直接的、不依赖DPP IV的作用,激活cAMP和ERK1/2信号传导,刺激GLP-1的总分泌[3]。 |
| 动物实验 |
Mice: C57BL/6J mice that have been fasted overnight are challenged with an oral glucose load (2 g/kg) 45 minutes after the compound is administered. Tail bleed predose and successive time points following the glucose load are used to draw blood samples for glucose measurement. DPP-4 inhibitors or a vehicle are given 16 hours prior to the glucose challenge in order to assess how long the effect lasts on glucose tolerance.
Effects of MK0431 on islet graft survival in diabetic NOD mice were determined with metabolic studies and micropositron emission tomography imaging, and its underlying molecular mechanisms were assessed. Results: Treatment of NOD mice with MK0431 before and after islet transplantation resulted in prolongation of islet graft survival, whereas treatment after transplantation alone resulted in small beneficial effects compared with nontreated controls. Subsequent studies demonstrated that MK0431 pretreatment resulted in decreased insulitis in diabetic NOD mice and reduced in vitro migration of isolated splenic CD4+ T-cells. Furthermore, in vitro treatment of splenic CD4+ T-cells with DPP-IV resulted in increased migration and activation of protein kinase A (PKA) and Rac1. Conclusions: Treatment with MK0431 therefore reduced the effect of autoimmunity on graft survival partially by decreasing the homing of CD4+ T-cells into pancreatic beta-cells through a pathway involving cAMP/PKA/Rac1 activation.[2] Effects of the DPP-IV inhibitor MK0431 (sitagliptin) on glycemic control and functional islet mass in a streptozotocin (STZ)-induced type 1 diabetes mouse model were determined with metabolic studies and microPET imaging. Results: The type 1 diabetes mouse model exhibited elevated plasma DPP-IV levels that were substantially inhibited in mice on an MK0431 diet. Residual beta-cell mass was extremely low in STZ-induced diabetic mice, and although active GLP-1 levels were increased by the MK0431 diet, there were no significant effects on glycemic control. After islet transplantation, mice fed normal diet rapidly lost their ability to regulate blood glucose, reflecting the suboptimal islet transplant. By contrast, the MK0431 group fully regulated blood glucose throughout the study, and PET imaging demonstrated a profound protective effect of MK0431 on islet graft size. Conclusions: Treatment with a DPP-IV inhibitor can prolong islet graft retention in an animal model of type 1 diabetes.[4] The pharmacokinetics, metabolism, and excretion of sitagliptin [MK-0431; (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine], a potent dipeptidyl peptidase 4 inhibitor, were evaluated in male Sprague-Dawley rats and beagle dogs. The plasma clearance and volume of distribution of sitagliptin were higher in rats (40-48 ml/min/kg, 7-9 l/kg) than in dogs ( approximately 9 ml/min/kg, approximately 3 l/kg), and its half-life was shorter in rats, approximately 2 h compared with approximately 4 h in dogs. Sitagliptin was absorbed rapidly after oral administration of a solution of the phosphate salt. The absolute oral bioavailability was high, and the pharmacokinetics were fairly dose-proportional. After administration of [(14)C]sitagliptin, parent drug was the major radioactive component in rat and dog plasma, urine, bile, and feces. Sitagliptin was eliminated primarily by renal excretion of parent drug; biliary excretion was an important pathway in rats, whereas metabolism was minimal in both species in vitro and in vivo. Approximately 10 to 16% of the radiolabeled dose was recovered in the rat and dog excreta as phase I and II metabolites, which were formed by N-sulfation, N-carbamoyl glucuronidation, hydroxylation of the triazolopiperazine ring, and oxidative desaturation of the piperazine ring followed by cyclization via the primary amine. The renal clearance of unbound drug in rats, 32 to 39 ml/min/kg, far exceeded the glomerular filtration rate, indicative of active renal elimination of parent drug.[5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Sitagliptin is 87% orally bioavailable and taking it with or without food does not affect its pharmacokinetics. Sitagliptin reaches maximum plasma concentration in 2 hours. Approximately 79% of sitagliptin is excreted in the urine as the unchanged parent compound. 87% of the dose is eliminated in the urine and 13% in the feces. 198L. 350mL/min. Sitagliptin is secreted in the milk of lactating rats at a milk to plasma ratio of 4:1. It is not known whether sitagliptin is excreted in human milk. Placental transfer of sitagliptin administered to pregnant rats was approximately 45% at 2 hours and 80% at 24 hours postdose. Placental transfer of sitagliptin administered to pregnant rabbits was approximately 66% at 2 hours and 30% at 24 hours. Approximately 79% of sitagliptin is excreted unchanged in the urine with metabolism being a minor pathway of elimination. Elimination of sitagliptin occurs primarily via renal excretion and involves active tubular secretion. Sitagliptin is a substrate for human organic anion transporter-3 (hOAT-3), which may be involved in the renal elimination of sitagliptin. The clinical relevance of hOAT-3 in sitagliptin transport has not been established. Sitagliptin is also a substrate of p-glycoprotein, which may also be involved in mediating the renal elimination of sitagliptin. However, cyclosporine, a p-glycoprotein inhibitor, did not reduce the renal clearance of sitagliptin. For more Absorption, Distribution and Excretion (Complete) data for SITAGLIPTIN (10 total), please visit the HSDB record page. Metabolism / Metabolites Sitagliptin is mostly not metabolised, with 79% of the dose excreted in the urine as the unchanged parent compound. Minor metabolic pathways are mediated mainly by cytochrome p450(CYP)3A4 and to a lesser extent by CYP2C8. After 18 hours, 81% of the dose has remained unchanged, while 2% has been N-sulfated to the M1 metabolite, 6% has been oxidatively desaturated and cyclized to the M2 metabolite, <1% glucuronidated at an unknown site to the M3 metabolite, <1% has been carbamoylated and glucuronidated to the M4 metabolite, 6% has been oxidatively saturated and cyclized to the M5 metabolite, and 2% has been hydroxylated at an unknown site to the M6 metabolite. The M2 metabolite is the cis isomer while the M5 metabolite is the trans isomer of the same metabolite. The metabolism and excretion of (14)C sitagliptin ... were investigated in humans after a single oral dose of 83 mg/193 muCi. Urine, feces, and plasma were collected at regular intervals for up to 7 days. The primary route of excretion of radioactivity was via the kidneys, with a mean value of 87% of the administered dose recovered in urine. Mean fecal excretion was 13% of the administered dose. Parent drug was the major radioactive component in plasma, urine, and feces, with only 16% of the dose excreted as metabolites (13% in urine and 3% in feces), indicating that sitagliptin was eliminated primarily by renal excretion. Approximately 74% of plasma AUC of total radioactivity was accounted for by parent drug. Six metabolites were detected at trace levels, each representing <1 to 7% of the radioactivity in plasma. These metabolites were the N-sulfate and N-carbamoyl glucuronic acid conjugates of parent drug, a mixture of hydroxylated derivatives, an ether glucuronide of a hydroxylated metabolite, and two metabolites formed by oxidative desaturation of the piperazine ring followed by cyclization. These metabolites were detected also in urine, at low levels. Metabolite profiles in feces were similar to those in urine and plasma, except that the glucuronides were not detected in feces. CYP3A4 was the major cytochrome P450 isozyme responsible for the limited oxidative metabolism of sitagliptin, with some minor contribution from CYP2C8. Following a (14)C sitagliptin oral dose, approximately 16% of the radioactivity was excreted as metabolites of sitagliptin. Six metabolites were detected at trace levels and are not expected to contribute to the plasma DPP-4 inhibitory activity of sitagliptin. In vitro studies indicated that the primary enzyme responsible for the limited metabolism of sitagliptin was CYP3A4, with contribution from CYP2C8. Biological Half-Life Approximately 12.4 hours. Other studies have reported a half life of approximately 11 hours. Two double-blind, randomized, placebo-controlled, alternating-panel studies evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of single oral doses of sitagliptin (1.5-600 mg) in healthy male volunteers. Sitagliptin was well absorbed (approximately 80% excreted unchanged in the urine) with an apparent terminal half-life ranging from 8 to 14 hours. ... The apparent terminal half life following a 100 mg oral dose of sitagliptin was approximately 12.4 hours ... . |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Sitagliptin is a viscous liquid. It is a dipeptidyl peptidase-4 inhibitor and used to improve glycemic control in patients with type 2 diabetes. HUMAN EXPOSURE AND TOXICITY: Sitagliptin improves glycemic control and is generally well-tolerated in patients with type 2 diabetes. Sitagliptin use has been associated with an increased risk of heart failure -related hospitalizations among patients with type 2 diabetes with pre-existing heart failure. More recently a study has pointed to the possible use of sitagliptin in the treatment of some neurodegenerative conditions of the peripheral nervous system. Sitagliptin appears to be free from the adverse effects of weight gain and hypoglycemia that are associated some other treatments. ANIMAL STUDIES: Renal and liver toxicity were observed in rodents at systemic exposure to sitagliptin at values 58 times the human exposure level. Transient treatment-related physical signs, some of which suggest neural toxicity, such as open-mouth breathing, salivation, white foamy emesis, ataxia, trembling, decreased activity, and/or hunched posture were observed in dogs at exposure levels approximately 23 times the clinical exposure level. Carcinogenicity studies in mice did not show an increased incidence of tumors in any organ up to 500 mg/kg, but in rats there was an increased incidence of combined liver adenoma/carcinoma in males and females and of liver carcinoma in females at 500 mg/kg. Reproductive effects in rats and rabbits were only seen at doses greater than 250 mg/kg. Incisor teeth abnormalities were observed in rats at exposure levels 67 times the clinical exposure level. Sitagliptin was not mutagenic or clastogenic with or without metabolic activation in the Ames bacterial mutagenicity assay, a Chinese hamster ovary (CHO) chromosome aberration assay, an in vitro cytogenetics assay in CHO cells, an in vitro rat hepatocyte DNA alkaline elution assay, and an in vivo micronucleus assay. Hepatotoxicity Liver injury due to sitagliptin is rare. In large clinical trials, serum enzyme elevations were no more common with sitagliptin therapy (0.5%) than with placebo (0.4%), and no instances of clinically apparent liver injury were reported. Since licensure, instances of serum enzyme elevations attributed to sitagliptin have been reported to the FDA and the sponsor. A single case report of clinically apparent liver injury has been published, but in a patient who also had hepatitis C. The pattern of serum enzyme elevations was hepatocellular and peak serum bilirubin was 9.4 mg/dL, with a rapid recovery upon stopping sitagliptin. Immunoallergic features and autoantibodies were absent. Likelihood score: D (possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sitagliptin during breastfeeding. Sitagliptin has a shorter half-life than most other dipeptidyl-peptidase IV inhibitors, so it might be a better choice among drugs in this class for nursing mothers. Monitoring of the breastfed infant's blood glucose is advisable during maternal therapy with sitagliptin. However, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 38%. Interactions Concomitant administration of cyclosporine and sitagliptin may increase absorption and plasma concentrations of sitagliptin. However, this interaction is not considered clinically important. Sitagliptin and metformin have a potential additive effect on active glucagon-like peptide (GLP-1) concentrations. Pharmacokinetic interactions are unlikely. The relevance of these effects to glycemic control in patients with type 2 diabetes mellitus is unclear. There was a slight increase in the area under the curve (AUC, 11%) and mean peak drug concentration (Cmax, 18%) of digoxin with the co-administration of 100 mg sitagliptin for 10 days. Patients receiving digoxin should be monitored appropriately. No dosage adjustment of digoxin or Januvia is recommended. When sitagliptin was used in combination with a sulfonylurea or insulin, the incidence of hypoglycemia was greater than that in patients receiving placebo with a sulfonylurea or insulin. In a long-term (52-week) clinical noninferiority study, rates of hypoglycemia with sitagliptin/metformin combination therapy were lower than those observed with glipizide/metformin combination therapy. However, in a 24-week clinical study, rates of hypoglycemia in patients receiving sitagliptin and glimepiride with or without metformin were greater than those in patients receiving glimepiride and metformin. Patients receiving sitagliptin may require a lower dosage of a concomitant insulin secretagogue (e.g., sulfonylurea) or insulin to reduce the risk of hypoglycemia. Non-Human Toxicity Values LD50 Mouse oral 4000 mg/kg LD50 Rat oral >3000 mg/kg |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Hypoglycemic Agents Januvia is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. /Included in US product label/ Januvia should not be used in patients with type 1 diabetes or for the treatment of diabetic ketoacidosis, as it would not be effective in these settings. Type 2 diabetes mellitus is a common chronic disease that causes significant morbidity and mortality worldwide. The primary goal of treatment is to target glycemic control by maintaining the glycosylated hemoglobin level near 6-7% without predisposing patients to hypoglycemia. Diabetes results from a combination of increased hepatic glucose production, decreased insulin secretion from beta cells, and insulin resistance in the peripheral tissues. Currently available antidiabetic agents work by different mechanisms to lower blood glucose levels. Unfortunately, each of them has its tolerability and safety concerns that limit its use and dose titration. Sitagliptin is the first antidiabetic agent from the class of dipeptidyl peptidase-4 enzyme inhibitors. It increases the amount of circulating incretins, which stimulate insulin secretion and inhibit glucose production. Sitagliptin was approved by the US Food and Drug Administration (FDA) for use with diet and exercise to improve glycemic control in adult patients with type 2 diabetes. It can be used alone or in combination with metformin or a thiazolidinedione (pioglitazone or rosiglitazone) when treatment with either drug alone provides inadequate glucose control. The usual adult dose is 100 mg once daily. A dose of 25-50 mg once daily is recommended for patients with moderate-to-severe renal impairment. In randomized, placebo-controlled trials that lasted for up to 6 months, sitagliptin lowered glycosylated hemoglobin levels by 0.5-0.8%. In a 52-week clinical trial, sitagliptin was shown to be noninferior to glipizide as an add-on agent in patients inadequately controlled on metformin alone. Sitagliptin was well tolerated with the most common side effects being gastrointestinal complaints (up to 16%), including abdominal pain, nausea and diarrhea; hypoglycemia and body weight gain occurred at similar rates compared with placebo. Overall, sitagliptin provides a treatment option for patients with type 2 diabetes as a monotherapy, or as an adjunct to metformin or a thiazolidinedione when patients achieve inadequate glycemic control while on either of the agents. It is also an alternative therapy for those patients who have contraindications or intolerability to other antidiabetic agents. For more Therapeutic Uses (Complete) data for SITAGLIPTIN (6 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: LACTIC ACIDOSIS. Lactic acidosis is a rare, but serious, complication that can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic impairment, renal impairment, and acute congestive heart failure. The onset of lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. Laboratory abnormalities include low pH, increased anion gap, and elevated blood lactate. If acidosis is suspected, Janumet should be discontinued and the patient hospitalized immediately. /Sitagliptin and metformin hydrochloride combination product/ FDA is evaluating unpublished new findings by a group of academic researchers that suggest an increased risk of pancreatitis and pre-cancerous cellular changes called pancreatic duct metaplasia in patients with type 2 diabetes treated with a class of drugs called incretin mimetics. These findings were based on examination of a small number of pancreatic tissue specimens taken from patients after they died from unspecified causes. FDA has asked the researchers to provide the methodology used to collect and study these specimens and to provide the tissue samples so the Agency can further investigate potential pancreatic toxicity associated with the incretin mimetics. Drugs in the incretin mimetic class include exenatide (Byetta, Bydureon), liraglutide (Victoza), sitagliptin (Januvia, Janumet, Janumet XR, Juvisync), saxagliptin (Onglyza, Kombiglyze XR), alogliptin (Nesina, Kazano, Oseni), and linagliptin (Tradjenta, Jentadueto). These drugs work by mimicking the incretin hormones that the body usually produces naturally to stimulate the release of insulin in response to a meal. They are used along with diet and exercise to lower blood sugar in adults with type 2 diabetes. FDA has not reached any new conclusions about safety risks with incretin mimetic drugs. This early communication is intended only to inform the public and health care professionals that the Agency intends to obtain and evaluate this new information. ... FDA will communicate its final conclusions and recommendations when its review is complete or when the Agency has additional information to report. The Warnings and Precautions section of drug labels and patient Medication Guides for incretin mimetics contain warnings about the risk of acute pancreatitis. FDA has not previously communicated about the potential risk of pre-cancerous findings of the pancreas with incretin mimetics. FDA has not concluded these drugs may cause or contribute to the development of pancreatic cancer. At this time, patients should continue to take their medicine as directed until they talk to their health care professional, and health care professionals should continue to follow the prescribing recommendations in the drug labels. ... Acute pancreatitis, including fatal and nonfatal hemorrhagic or necrotizing pancreatitis, has been reported during postmarketing experience in patients receiving sitagliptin or sitagliptin/metformin. The most common manifestations associated with pancreatitis were abdominal pain, nausea, and vomiting. Hospitalization was required in 66% of 88 reported cases, including 2 cases of hemorrhagic or necrotizing pancreatitis that necessitated prolonged hospitalization and intensive-care unit (ICU) care. Pancreatitis occurred within 30 days of initiation of sitagliptin or sitagliptin/metformin therapy in 21% of cases; discontinuance of the drug led to resolution of pancreatitis in 53% of patients. At least one other risk factor (e.g., obesity, high cholesterol and/or triglyceride concentrations) was noted in 51% of cases. Renal function should be assessed prior to initiation of sitagliptin and periodically thereafter. Worsening of renal function, including acute renal failure that sometimes required dialysis, has been reported in some patients during postmarketing experience. A subset of these patients had renal insufficiency, some of whom were prescribed inappropriate dosages of sitagliptin. A return to baseline levels of renal insufficiency has been observed with supportive treatment and discontinuance of potentially causative agents. Cautious reinitiation of sitagliptin can be considered if another etiology is deemed likely to have precipitated the acute worsening of renal function. The manufacturer states that sitagliptin has not been found to be nephrotoxic in clinical trials or in preclinical studies at clinically relevant dosages. For more Drug Warnings (Complete) data for SITAGLIPTIN (17 total), please visit the HSDB record page. Pharmacodynamics Sitagliptin inhibits DPP-4 which leads to increased levels of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide(GIP), decreased levels of glucagon, and a stronger insulin response to glucose. |
| 分子式 |
C16H15F6N5O
|
|---|---|
| 分子量 |
407.32
|
| 精确质量 |
407.118
|
| 元素分析 |
C, 47.18; H, 3.71; F, 27.99; N, 17.19; O, 3.93
|
| CAS号 |
486460-32-6
|
| 相关CAS号 |
Sitagliptin phosphate;654671-78-0;Sitagliptin phosphate monohydrate;654671-77-9;(S)-Sitagliptin phosphate;823817-58-9;(Rac)-Sitagliptin;823817-56-7;Sitagliptin-d4 hydrochloride
|
| PubChem CID |
4369359
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 沸点 |
529.9±60.0 °C at 760 mmHg
|
| 闪点 |
274.3±32.9 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.590
|
| LogP |
1.3
|
| tPSA |
77.04
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
566
|
| 定义原子立体中心数目 |
1
|
| SMILES |
FC(C1=NN=C2C([H])([H])N(C(C([H])([H])[C@@]([H])(C([H])([H])C3=C([H])C(=C(C([H])=C3F)F)F)N([H])[H])=O)C([H])([H])C([H])([H])N21)(F)F
|
| InChi Key |
MFFMDFFZMYYVKS-SECBINFHSA-N
|
| InChi Code |
InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1
|
| 化学名 |
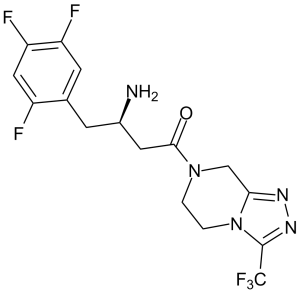
(3R)-3-amino-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one
|
| 别名 |
EC 690-730-1; HSDB 7516; HSDB7516; HSDB-7516; Januvia; LEZ 763; LEZ-763; LEZ763; Tesavel; Xelevia; (R)-3-AMINO-1-(3-(TRIFLUOROMETHYL)-5,6-DIHYDRO-[1,2,4]TRIAZOLO[4,3-A]PYRAZIN-7(8H)-YL)-4-(2,4,5-TRIFLUOROPHENYL)BUTAN-1-ONE; sitagliptina; MK-0431; MK0431; MK 0431; MK-431; MK431; MK 431; Sitagliptin Phosphate; Sitagliptin Phosphate Monohydrate; trade name: Januvia Xelevia Janumet
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.14 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.14 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.5 mg/mL (6.14 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 需要加热至 60°C。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4551 mL | 12.2754 mL | 24.5507 mL | |
| 5 mM | 0.4910 mL | 2.4551 mL | 4.9101 mL | |
| 10 mM | 0.2455 mL | 1.2275 mL | 2.4551 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Anti-Diabetic Medications to Fight PD and LBD
CTID: NCT06263673
Phase: Phase 4 Status: Recruiting
Date: 2024-08-15