| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g | |||
| Other Sizes |

| 靶点 |
Natural product; CYP2E1 (IC50 = 12.6 μM); NF-κB
- Reactive oxygen species (ROS): Scavenges intracellular ROS and inhibits ROS-mediated oxidative stress [4] - Inflammatory mediators: Modulates the production of pro-inflammatory cytokines (e.g., TNF-α, IL-6) without direct binding to cytokine receptors [4] - Antioxidant enzymes: Upregulates the activity of endogenous antioxidant enzymes (e.g., superoxide dismutase (SOD), catalase (CAT)) without targeting the enzymes themselves [1] . |
|---|---|
| 体外研究 (In Vitro) |
丹参素钠是丹参素的单钠盐,丹参素是从丹参中分离出来的化合物。丹参素是一种高效的自由基清除剂和抗氧化剂,对自由基(HO())、超氧阴离子自由基(O(2)(-))、1,1-二苯基-2-苦基肼(DPPH)具有较高的清除活性自由基和 2-连氮基-双(3-乙基苯并噻唑啉-6-磺酸) (ABTS) 自由基比维生素 C 强。丹参素钠对血管张力显示出双相作用。在有或没有内皮的去氧肾上腺素预收缩的胸动脉中,低浓度(0.1-0.3 g/L)丹参素钠产生微弱的收缩,而高浓度(1-3 g/L)在短暂的血管收缩后产生明显的血管舒张作用。丹参素钠预孵育可抑制去氧肾上腺素和氯化钾引起的血管收缩,且呈浓度依赖性。 Sodium danshensu 还可抑制无 Ca(2+) 培养基中去氧肾上腺素和 CaCl2 诱导的血管收缩。细胞测定:丹参素钠对血管张力显示出双相效应。低剂量的丹参素钠可能通过短暂增强Ca2+内流而产生小幅收缩,而高剂量则主要通过促进血管平滑肌细胞中非选择性K+通道和小电导钙敏感K+通道的开放而产生显着的血管舒张
1. 培养细胞中的抗氧化活性: - 在过氧化氢(H₂O₂,200 μM)诱导氧化应激的人脐静脉内皮细胞(HUVECs)中,Sodium Danshensu(50、100、200 μM)共孵育24小时,可使细胞内ROS水平较H₂O₂单独处理组分别降低32.5%±4.1%、56.2%±3.8%、71.8%±2.9%(DCFH-DA荧光探针检测);此外,200 μM Sodium Danshensu可使SOD活性升高1.8倍,CAT活性升高1.6倍(vs. H₂O₂组)[4] - 在四氯化碳(CCl₄,10 mM)暴露的大鼠原代肝细胞中,Sodium Danshensu(10、50、100 μM)以剂量依赖性方式抑制脂质过氧化(丙二醛MDA含量检测):MDA水平从模型组的2.8 nmol/mg蛋白分别降至2.1、1.5、1.1 nmol/mg蛋白[1] 2. 巨噬细胞中的抗炎活性: - 在脂多糖(LPS)刺激的RAW 264.7巨噬细胞中,Sodium Danshensu(25、50、100 μM)处理18小时,可使TNF-α分泌量较LPS单独处理组分别降低28.3%±3.5%、45.1%±2.7%、62.4%±4.2%,IL-6分泌量分别降低24.5%±2.9%、39.8%±3.1%、57.6%±3.8%(ELISA检测);RT-PCR结果显示,100 μM Sodium Danshensu可使iNOS mRNA表达降低48.7%,COX-2 mRNA表达降低42.3%[4] 3. 抗氧化损伤的细胞保护作用: - 在H₂O₂诱导的HUVEC凋亡模型中(Annexin V-FITC/PI染色),100 μM Sodium Danshensu可使凋亡率从模型组的35.6%±3.2%降至12.3%±2.1%;Western blot显示,50-200 μM Sodium Danshensu可使Bcl-2表达上调1.5-2.2倍,Bax表达下调0.6-0.3倍(vs. H₂O₂组)[4] 。 |
| 体内研究 (In Vivo) |
分离正常大鼠的胸主动脉,并在带有 Krebs-Henseleit 缓冲液的器官浴中平衡,并记录环张力。观察丹参素钠对血管基础张力的影响及其对有内皮或无内皮血管收缩和舒张的影响。体内:丹参素不改变糖尿病小鼠海马中AGEs的表达,但部分阻断RAGE表达的增加。丹参素可以通过减轻晚期糖基化终产物介导的神经炎症来改善链脲佐菌素诱导的糖尿病小鼠的认知能力下降
1. CCl₄诱导肝损伤小鼠中的抗氧化及保肝作用: - 雄性ICR小鼠(20-25 g)腹腔注射CCl₄(0.2 mL/kg,50%橄榄油溶液)诱导急性肝损伤;Sodium Danshensu以50、100、200 mg/kg剂量灌胃给药,每日1次,连续7天(CCl₄注射前1天至注射后5天)。治疗结束时: - 血清肝功能指标:ALT水平从模型组的586 U/L分别降至421、298、185 U/L;AST水平从模型组的452 U/L分别降至345、256、168 U/L[1] - 肝脏抗氧化指标:肝组织SOD活性从模型组的85 U/mg蛋白分别升至112、145、178 U/mg蛋白;MDA含量从模型组的3.2 nmol/mg蛋白分别降至2.5、1.8、1.2 nmol/mg蛋白[1] - 组织病理学:200 mg/kg Sodium Danshensu可减轻CCl₄诱导的肝细胞坏死和炎症细胞浸润(HE染色)[1] 2. 大鼠体内药代动力学分布: - 雄性SD大鼠(250-300 g)分为静脉注射(i.v.)和口服(p.o.)两组: - 静脉组:尾静脉单次注射10 mg/kg Sodium Danshensu(生理盐水溶解),于给药后0.083、0.25、0.5、1、2、4、6、8小时采血。血药浓度-时间曲线符合二室模型,分布半衰期(t₁/₂α)=0.21±0.05 h,消除半衰期(t₁/₂β)=2.34±0.28 h,峰浓度(Cmax)=1256±132 ng/mL,药时曲线下面积(AUC₀-∞)=1580±145 ng·h/mL[3] - 口服组:灌胃单次给予40 mg/kg Sodium Danshensu(0.5%羧甲基纤维素溶解),于给药后0.25、0.5、1、2、3、4、6、8小时采血。药代参数:达峰时间(Tmax)=1.0±0.2 h,Cmax=328±45 ng/mL,AUC₀-∞=1180±120 ng·h/mL,口服生物利用度(F)=18.7%±2.3%[3] - 组织分布:静脉注射10 mg/kg Sodium Danshensu后0.5小时,各组织药物浓度(ng/g)为:肾脏(456)>肝脏(328)>心脏(285)>脑(12)(UHPLC-MS/MS检测)[3] 。 |
| 酶活实验 |
丹红注射液(DHI)是一种中成药,主要用于治疗缺血性脑病和冠心病,并与其他化疗联合使用。然而,关于DHI潜在药物相互作用的信息是有限的。这项工作的目的是研究DHI及其活性成分引起的潜在P450介导的代谢药物相互作用。结果显示,DHI抑制CYP2C19、CYP2D6、CYP3A4、CYP2E1和CYP2C9,IC50值分别为1.26、1.42、1.63、1.10和1.67%(v/v)。丹参素和迷迭香酸抑制CYP2E1和CYP2C9,IC50值分别为36.63和75.76μm,34.42和76.89μm。丹酚酸A和B抑制CYP2D6、CYP2E1和CYP2C9,IC50值分别为33.79、21.64和31.94μm,45.47、13.52和24.15μm。该研究为DHI在临床实践中的安全有效使用提供了一些有用的信息[3]。
1. SOD活性检测: - 制备10%(w/v)肝组织匀浆或细胞裂解液(冰生理盐水),取0.1 mL匀浆/裂解液与2.9 mL含黄嘌呤、黄嘌呤氧化酶、氮蓝四唑(NBT)的反应缓冲液混合,37℃孵育40分钟,在550 nm波长测吸光度。SOD活性定义为抑制50% NBT还原所需的酶量,单位为U/mg蛋白。Sodium Danshensu处理组的吸光度抑制率更高(提示SOD活性更高)[1,4] 2. CAT活性检测: - 取0.2 mL组织匀浆/细胞裂解液,与1.8 mL 0.067 M磷酸缓冲液(pH 7.4)和1 mL 0.03 M H₂O₂混合,记录1分钟内240 nm吸光度的下降值。CAT活性根据H₂O₂分解速率计算,单位为U/mg蛋白(1 U定义为每分钟分解1 μmol H₂O₂)。100-200 μM Sodium Danshensu可使H₂O₂诱导的HUVECs中CAT活性升高1.4-1.6倍[4] 。 |
| 细胞实验 |
通过用“缺血缓冲液”替代培养基,“缺血缓冲液”模拟心肌缺血的细胞外环境,并且含有与体内发现的浓度相似的钾、氢和乳酸离子浓度,使心肌细胞遭受缺血。使用含有 5% CO2 和 95% 氮气的潮湿气氛在缺氧/缺血室中在 37°C 下孵育细胞两小时。再灌注开始时,心肌细胞随机接受以下治疗之一:载体、丹参素(1 或 10 μM)、丹参素加 PI3K 抑制剂渥曼青霉素(10 nM)或丹参素加 ERK 抑制剂 U0126(10 μM)。 H9c2心肌细胞在CO2培养箱中正常培养,同时对照组心肌细胞。
1. HUVEC氧化应激与凋亡实验: - 细胞接种:HUVECs以5×10³个/孔(96孔板,ROS/MDA检测)或2×10⁵个/孔(6孔板,凋亡/Western blot)接种,用含10% FBS的RPMI 1640培养基在37℃、5% CO₂条件下培养24小时[4] - 氧化应激诱导与药物处理:更换为含200 μM H₂O₂及Sodium Danshensu(25、50、100、200 μM)或溶媒(生理盐水)的培养基,继续孵育24小时[4] - ROS检测:细胞与10 μM DCFH-DA探针37℃孵育30分钟,PBS洗涤后,用酶标仪在激发波长488 nm、发射波长525 nm处检测荧光强度,以相对荧光单位(RFU)表示ROS水平[4] - 凋亡检测:胰酶消化细胞,PBS洗涤后,Annexin V-FITC和PI避光染色15分钟,流式细胞仪分析,凋亡率以Annexin V阳性细胞百分比表示[4] - Western blot:含蛋白酶抑制剂的RIPA裂解液裂解细胞,30 μg蛋白经SDS-PAGE分离后转移至PVDF膜,用抗Bcl-2、抗Bax、抗β-actin抗体孵育,ImageJ定量条带强度[4] 2. RAW 264.7巨噬细胞炎症实验: - RAW 264.7细胞以1×10⁵个/孔接种于24孔板,含10% FBS的DMEM培养;Sodium Danshensu(25、50、100 μM)预处理2小时后,加入1 μg/mL LPS刺激18小时[4] - 细胞因子检测:收集培养上清,ELISA试剂盒检测TNF-α/IL-6浓度,450 nm测吸光度,标准曲线计算细胞因子水平[4] - RT-PCR:TRIzol提取总RNA,逆转录为cDNA后,用iNOS/COX-2特异性引物进行PCR扩增,GAPDH为内参,琼脂糖凝胶电泳分析产物并定量条带强度[4] 。 |
| 动物实验 |
Paeonol (80 mg kg(-1)) and danshensu (160 mg kg(-1)) were administered orally to Sprague Dawley rats in individual or in combination for 21 days. At the end of this period, rats were administered isoproterenol (85 mg kg(-1)) subcutaneously to induce myocardial injury. After induction, rats were anaesthetized with pentobarbital sodium (35 mg kg(-1)) to record electrocardiogram, then sacrificed and biochemical assays of the heart tissues were performed. Principal findings: Induction of rats with isoproterenol resulted in a marked (P<0.001) elevation in ST-segment, infarct size, level of serum marker enzymes (CK-MB, LDH, AST and ALT), cTnI, TBARS, protein expression of Bax and Caspase-3 and a significant decrease in the activities of endogenous antioxidants (SOD, CAT, GPx, GR, and GST) and protein expression of Bcl-2. Pretreatment with paeonol and danshensu combination showed a significant (P<0.001) decrease in ST-segment elevation, infarct size, cTnI, TBARS, protein expression of Bax and Caspase-3 and a significant increase in the activities of endogenous antioxidants and protein expression of Bcl-2 and Nrf2 when compared with individual treated groups.[4]
1. CCl₄-induced acute liver injury mouse model (hepatoprotective study): - Animals: Male ICR mice (20-25 g, 6-8 weeks old) were randomly divided into 5 groups (n=8 per group): Normal control, CCl₄ model, Sodium Danshensu low dose (50 mg/kg), middle dose (100 mg/kg), high dose (200 mg/kg) [1] - Drug preparation and administration: Sodium Danshensu was dissolved in 0.5% carboxymethylcellulose (CMC) to prepare suspensions of different concentrations. All doses were administered by intragastric gavage (0.2 mL/10 g body weight) once daily. The normal control and CCl₄ model groups received equal volumes of 0.5% CMC [1] - Liver injury induction: On day 2 of administration, the CCl₄ model group and Sodium Danshensu groups were intraperitoneally injected with CCl₄ (0.2 mL/kg, 50% v/v in olive oil); the normal control group received olive oil alone [1] - Sample collection and detection: On day 8 (7 days of administration), mice were fasted for 12 hours, anesthetized with ether, and blood was collected via orbital vein to detect serum ALT/AST. Livers were excised: a portion was fixed in 4% formalin for HE staining; another portion was homogenized to detect SOD activity and MDA content [1] 2. Rat pharmacokinetic study: - Animals: Male SD rats (250-300 g, 8-10 weeks old) were randomly divided into 2 groups (i.v. and p.o., n=6 per group) and fasted for 12 hours before administration (free access to water) [3] - Drug preparation: For i.v. administration, Sodium Danshensu was dissolved in normal saline to a concentration of 2 mg/mL; for p.o. administration, it was dissolved in 0.5% CMC to a concentration of 8 mg/mL [3] - Administration and sampling: - I.v. group: Rats received a single tail vein injection of 10 mg/kg Sodium Danshensu (5 mL/kg volume). Blood samples (0.5 mL) were collected from the orbital venous plexus at 0.083, 0.25, 0.5, 1, 2, 4, 6, 8 hours post-administration, placed in heparinized tubes, and centrifuged at 3000 × g for 10 minutes to obtain plasma [3] - P.o. group: Rats received a single oral gavage of 40 mg/kg Sodium Danshensu (5 mL/kg volume). Blood samples were collected at 0.25, 0.5, 1, 2, 3, 4, 6, 8 hours post-administration, processed as above [3] - Tissue distribution: A separate group of rats (n=3 per time point) received i.v. 10 mg/kg Sodium Danshensu and were euthanized at 0.5, 2, 4 hours post-administration. Heart, liver, kidney, brain, and lung were excised, rinsed with cold saline, blotted dry, weighed, and homogenized in normal saline (1:4, w/v). Plasma and tissue homogenates were stored at -80°C until UHPLC-MS/MS analysis [3] . |
| 药代性质 (ADME/PK) |
1. Absorption:
- Oral absorption of Sodium Danshensu in SD rats is moderate: after a single p.o. dose of 40 mg/kg, Tmax = 1.0 ± 0.2 h, Cmax = 328 ± 45 ng/mL, and oral bioavailability (F) = 18.7% ± 2.3% (calculated by comparing AUC₀-∞ of p.o. and i.v. groups) [3] - Food effect: No significant effect of food on oral absorption was reported in the included literatures [3] 2. Distribution: - After i.v. administration of 10 mg/kg Sodium Danshensu in rats, the drug distributes rapidly to peripheral tissues (t₁/₂α = 0.21 ± 0.05 h). At 0.5 h post-administration, tissue concentrations (ng/g) are ranked as: kidney (456 ± 38) > liver (328 ± 25) > heart (285 ± 22) > lung (210 ± 18) > brain (12 ± 3) (low brain penetration due to blood-brain barrier) [3] 3. Metabolism: - In vitro liver microsome assay (human and rat): Sodium Danshensu (10 μM) was incubated with liver microsomes and NADPH for 2 hours. No major metabolites were detected by UHPLC-MS/MS, suggesting low hepatic metabolism [3] 4. Elimination: - In rats, Sodium Danshensu is eliminated mainly via the renal route. After i.v. administration of 10 mg/kg, the elimination half-life (t₁/₂β) = 2.34 ± 0.28 h, clearance (CL) = 6.32 ± 0.58 mL/kg/min, and volume of distribution at steady state (Vdss) = 1.05 ± 0.12 L/kg [3] - Within 8 hours post-i.v. administration, approximately 35% ± 4% of the dose was excreted as unchanged drug in urine [3] . |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute toxicity in mice:
- Male ICR mice (20-25 g) were administered a single oral dose of Sodium Danshensu (1000, 2000, 4000 mg/kg). No mortality was observed within 14 days. Body weight, food intake, and water intake were not significantly different from the normal control group. Serum biochemical parameters (ALT, AST, BUN, Cr) and organ weights (liver, kidney, heart) showed no abnormal changes [1] - The median lethal dose (LD₅₀) of oral Sodium Danshensu in mice was determined to be >4000 mg/kg, indicating low acute toxicity [1] 2. Subacute toxicity in mice: - Mice were administered Sodium Danshensu (50, 200, 800 mg/kg/day) via oral gavage for 28 days. No significant changes in body weight, organ coefficients (liver/body weight, kidney/body weight), or hematological parameters (RBC, WBC, PLT, Hb) were observed. Serum ALT, AST, BUN, and Cr levels were within the normal range. Histopathological examination of liver and kidney showed no obvious lesions (e.g., necrosis, fibrosis) [1] 3. Cytotoxicity in vitro: - In HUVECs and RAW 264.7 macrophages, Sodium Danshensu at concentrations up to 400 μM had no significant effect on cell viability (MTT assay: viability >90% vs. control), indicating no direct cytotoxicity [4] . |
| 参考文献 |
[1]. Food Chem Toxicol.2008 Jan;46(1):73-81;
[2]. Toxicol Mech Methods.2010 Oct;20(8):510-4. [3]. Biomed Chromatogr. 2018 Aug;32(8):e4250. [4]. PLoS One. 2012;7(11):e48872. |
| 其他信息 |
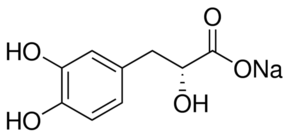
Sodium danshensu is a monocarboxylic acid and a member of benzenes.
1. Background and source: - Sodium Danshensu is the sodium salt of Danshensu (3-(3,4-dihydroxyphenyl)lactic acid), a major water-soluble active component isolated from the root of Salvia miltiorrhiza Bunge (Danshen), a traditional Chinese medicine widely used for cardiovascular and hepatic disorders [1,3,4] 2. Mechanism of action: - Antioxidant mechanism: Sodium Danshensu scavenges free radicals (e.g., ·OH, O₂⁻) directly via its catechol structure and upregulates endogenous antioxidant enzymes (SOD, CAT) to enhance oxidative stress resistance [1,4] - Anti-inflammatory mechanism: It inhibits LPS-induced activation of the NF-κB signaling pathway (by reducing IκBα phosphorylation) to downregulate pro-inflammatory cytokines (TNF-α, IL-6) and inflammatory enzymes (iNOS, COX-2) [4] - Hepatoprotective mechanism: By inhibiting CCl₄-induced lipid peroxidation (reducing MDA) and enhancing hepatic antioxidant capacity (increasing SOD/CAT), it alleviates hepatic oxidative damage and necrosis [1] 3. Therapeutic potential: - Sodium Danshensu shows potential for treating oxidative stress-related diseases, such as non-alcoholic fatty liver disease (NAFLD), cardiovascular diseases (e.g., atherosclerosis), and inflammatory disorders [1,4] - Its good safety profile (low acute/subacute toxicity) and moderate oral bioavailability support its development as a therapeutic agent [1,3] ; |
| 分子式 |
C9H9O5.NA
|
|
|---|---|---|
| 分子量 |
220.15
|
|
| 精确质量 |
220.034
|
|
| 元素分析 |
C, 49.10; H, 4.12; Na, 10.44; O, 36.34
|
|
| CAS号 |
67920-52-9
|
|
| 相关CAS号 |
Danshensu;76822-21-4;(Rac)-Salvianic acid A;23028-17-3
|
|
| PubChem CID |
23711819
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
-0.3
|
|
| tPSA |
100.82
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
15
|
|
| 分子复杂度/Complexity |
211
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
ZMMKVDBZTXUHFO-UHFFFAOYSA-M
|
|
| InChi Code |
InChI=1S/C9H10O5.Na/c10-6-2-1-5(3-7(6)11)4-8(12)9(13)14;/h1-3,8,10-12H,4H2,(H,13,14);/q;+1/p-1
|
|
| 化学名 |
sodium;3-(3,4-dihydroxyphenyl)-2-hydroxypropanoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (11.36 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (11.36 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 50 mg/mL (227.12 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5424 mL | 22.7118 mL | 45.4236 mL | |
| 5 mM | 0.9085 mL | 4.5424 mL | 9.0847 mL | |
| 10 mM | 0.4542 mL | 2.2712 mL | 4.5424 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|
|
|