| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Natural product
|
|---|---|
| 体外研究 (In Vitro) |
(Rac)-丹参素 ((Rac)-Danshensu) 比维生素 C 更有效地清除自由基、超氧阴离子自由基 (O2)、1,1-二苯基-2-苦基肼 (DPPH) 自由基和 2 -嗪基-双(3-乙基苯并噻唑啉-6-磺酸) (ABTS) 自由基[1]。
丹参素(3-(3,4-二羟基苯基)乳酸)和丹酚酸B是从丹参根中分离出来的咖啡酸衍生物中的两种天然酚酸,已被报道对氧化损伤具有潜在的保护作用。丹参根是治疗各种心血管疾病最广泛的中药。为了更好地了解它们的生物学功能,我们将丹参素和丹酚酸B与维生素c一起进行了体外自由基清除和抗氧化活性的研究。丹参素和丹酚酸B对游离羟基自由基(HO())、超氧阴离子自由基(O(2)(-))、1,1-二苯基-2-苦酰肼(DPPH)自由基和2-氮基-双(3-乙基苯并噻唑-6-磺酸)(ABTS)自由基的清除活性均高于维生素c。丹参素和丹酚酸B的铁螯合和过氧化氢(H(2)O(2))清除能力弱于维生素C。以维生素C当量(VCEAC)表示,ABTS自由基测定的相对VCEAC值(mg/100ml)依次为丹酚酸B(18.59) >丹参素(12.89)>维生素C(10.00)。过氧化氢对人静脉血管内皮细胞损伤的保护作用与其抗氧化活性相关。对这两种化合物的构效关系分析表明,丹参素与咖啡酸的缩合结合对其抗氧化活性具有重要作用。结果表明,丹参素和丹酚酸B都是有效的自由基清除剂和抗氧化剂,且丹酚酸B优于丹参素。其自由基清除和抗氧化性能在食品和医疗保健行业具有潜在的应用前景。[1] Danshensu/丹参素体外对SARS-CoV-2和S蛋白伪型病毒的影响[3] 如图1a, e所示,丹参素显示出较强的抗病毒活性,EC50为0.97 μM,对SARS-CoV-2的抑制呈浓度依赖性。丹参素还能有效抑制sars - cov - 2s蛋白伪型病毒(sars - cov - 2s)进入ace2过表达的HEK-293T细胞(IC50 = 0.31 μM)和Vero-E6细胞(IC50 = 4.97 μM)(图1b, c),但对VSV-G伪病毒无明显抑制作用(图1d)。S蛋白只能从含有SARS-CoV-2 S的上清液中检测到,以确认伪型SARS-CoV-2,如图1f所示。总之,丹参素已显示出对SARS-CoV-2的潜在抗病毒能力。 背景:口腔鳞状细胞癌(OSCC)约占所有口腔癌病例的90%,由于其高度转移性,预后较差。大多数OSCC治疗方案都有有害的副作用。假设/目的:本研究旨在研究具有生物活性的植物化学物质钠丹参素对人口腔癌细胞转移的影响。方法与结果:不同剂量丹参素钠(25 μM、50 μM、100 μM)处理FaDu和Ca9-22细胞后,与未处理的细胞相比,FaDu和Ca9-22细胞的运动性、迁移性和侵袭性明显降低。这种效应与MMP-2、vimentin和N-cadherin的表达降低以及E-cadherin和ZO-1的表达增强有关。进一步的分子机制研究表明,丹参素钠处理可显著降低p38磷酸化水平;然而,ERK1/2的磷酸化仅在FaDu细胞中显著降低,而p-JNK1/2未显示任何改变。p38和JNK1/2抑制剂与丹参素钠联合使用也减少了FaDu和Ca9-22细胞系的迁移。结论:丹参素钠通过抑制口腔癌p38磷酸化发挥抗转移作用。本研究确定丹参素钠是一种潜在的天然抗癌药物,可用于治疗高转移性OSCC。 丹参素/Danshensu对顺铂诱导的氧化应激的影响[5] 通过顺铂和HK-2细胞致小鼠肾损伤实验验证丹参素的抗氧化作用。脂质过氧化物如MDA被认为是ROS损伤的一种容易获得的生物标志物。此外,细胞还产生了多种内源性抗氧化酶(如SOD、CAT和GSH-Px)来解毒ROS,防止氧化应激。通过观察丹参素对体内外顺铂诱导的氧化应激的影响,检测总活性氧(tROS)、丙二醛(MDA)和抗氧化酶的变化。在体内,与对照组相比,顺铂显著增加肾脏组织中tROS和MDA的水平。丹参素组在三次剂量下均可降低tROS和MDA水平(图3A和B)。同时,顺铂组肾脏抗氧化酶活性降低。与顺铂组相比,丹参素处理组肾脏组织中抗氧化酶活性升高(图3C-E)。 体外采用顺铂刺激HK-2细胞建立细胞模型,评价丹参素对细胞活性及活性氧产生的影响。首先进行细胞毒性试验。证实丹参素浓度在2.5 ~ 20 μM之间无细胞毒性,顺铂的IC50为20 μM(图4A和B)。结果显示,丹参素在5 μM和10 μM浓度下均能减弱顺铂的细胞毒性(图4C)。顺铂孵育的HK-2细胞中ROS水平也较高,而丹参素在10 μM剂量下可降低ROS水平(图4D)。由此可见,丹参素对小鼠和顺铂引起的HK-2细胞氧化应激均有抗氧化作用。 丹参素/Danshensu通过Nrf2/ HO-1通路对氧化应激的影响[5] 研究发现Nrf-2是机体抗氧化应激的重要调节因子。通过检测Nrf2、HO-1和NQO1的表达,探讨丹参素通过激活Nrf2/ HO-1通路抗顺铂诱导氧化应激的机制。如图5所示,与对照组相比,顺铂组肾组织和HK-2细胞中Nrf2、HO-1和NQO1的表达均升高。丹参素组剂量依赖性上调Nrf2和HO-1的表达,激活Nrf2/ HO-1通路。 |
| 体内研究 (In Vivo) |
在兔子中,(Rac)-丹酚酸 A((Rac)-Danshensu;静脉注射;30 mg/kg)的 T1/2 为 16.6 分钟。小鼠经气管注射sars - cov - 2s诱导ALI,而VSV-G治疗小鼠作为对照。小鼠感染sars - cov - 2s前分别给予Danshensu/丹参素(25、50、100 mg/kg,静脉注射,1次)或丹参素(25、50、100 mg·kg-1·d-1,口服,连用7 d)。结果表明,sars - cov - 2s感染诱导严重的炎症细胞浸润,严重破坏肺组织结构,炎症细胞因子高表达,激活TLR4和NF-κB p65的高磷酸化;肺组织中血管紧张素原(AGT) mRNA高表达,ACE2 mRNA低表达。口服和静脉注射丹参素均能剂量依赖性地减轻SARS-CoV-2 S感染小鼠的病理改变。本研究不仅建立了伪型SARS-CoV-2 (SARS-CoV-2 S)诱导的ALI小鼠模型,而且还证明丹参素是抑制COVID-19患者肺部炎症反应的潜在治疗方法。[3]
丹参素可预防sars - cov - 2s蛋白诱导的急性肺部炎症[3] 采用H&E染色观察丹参素对生理损伤的保护作用。如图3所示,H&E染色后显微镜下观察各组小鼠肺组织病理变化。VSV-G组小鼠肺泡结构正常,无出血、肺泡间隙缩小、肺泡隔明显增厚、炎症细胞浸润。建立模型后,sars - cov - 2s组小鼠肺泡隔增厚,肺泡塌陷缩小,炎症细胞浸润明显。丹参素可明显减轻这些变化,且呈剂量依赖性。丹参素50和100 mg/kg组肺泡隔轻度增厚,可见少量炎症细胞,部分肺泡外观萎缩(图3c, d)。SARS-CoV-2 S组肺组织学评分显著高于空白组和VSV-G组,丹参素50、100 mg/kg组和丹参素1、100 mg/kg组肺组织学评分显著低于SARS-CoV-2 S组(图3a, d)(均P < 0.05)。分析结果表明,丹参素可明显改善SARS-CoV-2诱导的急性肺部炎症的组织病理状况。 丹参素/Danshensu可改善血清和肺组织炎症因子[3] 采用ELISA试剂盒检测丹参素对血清白细胞介素-6、白细胞介素-1β、肿瘤坏死因子-α的影响。如图4a-c所示,与空白组和VSV-G组相比,sars - cov - 2s刺激后血清样品中TNF-α、IL-1β和IL-6水平明显升高。然而,与sars - cov - 2s组相比,丹参素显著降低了血清中TNF-α、IL-1β和IL-6的水平。 各组小鼠肺组织检测TNF-α、IL-1β、IL-6 mRNA表达,见图4d-f。丹参素50和100 mg/kg可显著降低TNF-α、IL-1β和IL-6,而SARS-CoV-2 S可升高这些促炎生物标志物的mRNA水平。 丹参素/Danshensu均可保护抗氧化系统 [3] 丹参素对氧化应激的影响见图4。sars - cov - 2s处理降低了CAT(图4h)和GPx(图4i)活性。与此相反,丹参素预处理提高了CAT和GPx的活性。 丹参素对sars - cov - 2s诱导肺组织中p-NF-κB p65和TLR4表达的调控 [3] TLR4是传统的NF-κB p65通路的调节受体,间接在上调TNF-α、IL-1β和IL-6中发挥重要作用。如图5a所示,经sars - cov - 2s处理的小鼠肺组织中TLR4和p-NF-κB p65的表达明显上调。而丹参素能有效抑制sars - cov - 2s处理小鼠肺组织中TLR4和NF-κB p65的激活。 丹参素可逆转SARS-CoV-2诱导的肺组织中ACE2、AGT和炎性细胞因子的mRNA表达[3] 采用qPCR对小鼠肺组织进行分析,以评估其对ACE2和AGT mRNA的诱导作用。在所有小鼠的肺中检测到ACE2和AGT mRNA的表达,如图5b, c所示。丹参素治疗显著降低AGT(图5c),而SARS-CoV-2 S升高AGT的mRNA水平。丹参素处理显著提高了ACE2 mRNA水平(图5b)。丹参素给药100 mg/kg和丹参素预处理能有效降低促炎基因AGT mRNA表达至正常水平,提高促炎基因ACE2 mRNA表达。丹参素50 mg/kg ig也可降低部分病例AGT mRNA的表达。 顺铂的临床应用主要受到严重肾毒性的限制。丹参素/Danshensu是从丹参根中提取的主要药理活性二萜类化合物。本研究旨在探讨丹参素对顺铂所致肾毒性的保护作用及其可能机制。禁食12 h后,除对照组外,其余各组小鼠均单次腹腔注射顺铂25 mg/kg。1 h后,顺铂组(25 mg/kg) +丹参素组(15 mg/kg、30 mg/kg、60 mg/kg)给予相应剂量丹参素,每天1次,连续7 d。测定血尿素氮(BUN)、肌酐、活性氧(ROS)、超氧化物歧化酶(SOD)、谷胱甘肽过氧化物酶(GPx)、过氧化氢酶(CAT)和丙二醛(MDA)。ELISA法检测炎症因子TNF-α、IL-6、IL-1β的表达。结果表明,丹参素能改善大鼠肾损伤,降低血清BUN、肌酐、细胞因子和氧化应激指标。进一步研究表明,丹参素可诱导Nrf2/HO-1活化,抑制NF-κB通路。综上所述,丹参素对顺铂所致肾毒性具有保护作用,其机制可能与激活Nrf2/HO-1、抑制NF-ĸB通路有关。[5] |
| 酶活实验 |
金属螯合活性测定[1]
对亚铁离子的螯合活性根据Dinis et al.(1994)的方法测定。反应混合物中含有0.5 ml不同浓度的试验化合物、1.6 ml去离子水和0.05 ml 2mm FeCl2溶液。30s后,加入0.1 ml的5mm亚铁锌溶液。Fe2+-亚铁锌品红配合物在水中具有良好的水溶性和稳定性。室温下10 min后,测定562 nm处吸光度。测试化合物对亚铁螯合的相对活性以吸光度消失的百分比(%)表示[(对照品−样品)/对照品× 100]。 过氧化氢清除活性测定[1] 过氧化氢清除活性用替代滴定法测定(Zhao et al., 2006)。将1.0 ml 0.1 mM H2O2溶液与1.0 ml不同浓度的试验化合物混合,然后滴入2滴3%钼酸铵、10 ml 2m H2SO4和7.0 ml 1.8 M KI。用5.09 mM的NaS2O3滴定混合溶液,直至黄色消失。受试化合物清除过氧化氢的相对活性以滴度体积变化的百分比(%)表示[(Vcontrol−Vsample)/Vcontrol × 100]。 羟基自由基清除活性测定[1] 清除羟基自由基活性的测定是基于Fenton反应(Yu et al., 2004)。反应混合物中含有0.02 mM FeCl2, 0.03 mM 1,10-菲罗啉,0.16 M磷酸盐缓冲液(pH 7.8), 8.5 mM H2O2和各种浓度的测试化合物。混合物的总体积为3ml。加入H2O2开始反应。室温孵育5min后,测定560nm处吸光度。相对羟基自由基清除活性的计算方法与金属螯合活性测定方法相似。 超氧阴离子清除活性测定[1] 超氧阴离子在非酶促PMS-NADH体系中生成,并根据Liu et al.(1997)的方法通过NBT还原来测量。取0.75 ml 120 μM PMS溶液加入3 ml 100 mM Tris-HCl缓冲液(pH 7.4)中,缓冲液中分别含有0.75 ml 300 μM NBT溶液、0.75 ml 936 μM NADH溶液和0.3 ml不同浓度的被试化合物。室温孵育5min后,测定560nm处吸光度。结果以超氧阴离子清除的百分比(%)表示,计算方法类似于金属螯合活性测定。 维生素C等效抗氧化能力(VCEAC)测定[1] 用DPPH自由基和ABTS自由基表示被试化合物的总抗氧化能力为VCEAC。VCEAC的测定和计算方法为Kim et al., 2002, Kim and Lee ., 2004 DPPH自由基清除活性测定。[1] 将2.9 ml 0.1 μM DPPH 80%甲醇溶液与0.1 ml不同浓度的试验化合物混合。将混合物在室温下摇匀,静置30分钟达到稳定状态。测定517 nm处吸光度,确定DPPH的脱色效果。 ABTS自由基清除活性测定。[1] 制备1.0 mM AAPH和2.5 mM ABTS的混合物,加入100 mM磷酸缓冲盐水(PBS)溶液(pH 7.4,含150 mM NaCl),在68°C水浴中培养13 min,生成着色的ABTS自由基。用PBS调节所得ABTS自由基溶液的浓度,使其在734 nm处的吸光度为0.650±0.020。取60 μl不同浓度的测试样品等分加入到2.94 ml ABTS自由基溶液中,37℃水浴反应10 min,测定734 nm处吸光度的下降。 生成ABTS自由基和DPPH自由基吸光度随维生素C浓度降低的标准曲线。不同浓度丹酚酸和丹参素/Danshensu对ABTS自由基和DPPH自由基的吸光度分别在734 nm和517 nm处下降。使用各自的维生素C标准曲线将它们转化为VCEAC。实验化合物对ABTS和DPPH自由基的清除活性以VCEAC (mg/100 ml)计算。 |
| 细胞实验 |
细胞培养和细胞活力测定采用MTT [1]
如前所述,通过II型胶原酶处理从新鲜获得的人脐带中分离出人脐静脉内皮细胞(Ding et al., 2005)。计数内皮细胞,以5 × 104个/孔的密度将其接种到明胶包被的96孔板上,该96孔板中含有添加15%胎牛血清、100 U/ml青霉素和100 μg/ml链霉素的内皮生长培养基,在含5% CO2的37℃湿化环境中培养。用不同浓度的丹酚酸或丹参处理4小时后,用0.4 mM H2O2处理18小时,每孔用PBS洗涤两次以去除培养基。每孔加入100微升MTT (0.5 mg/ml), 37℃孵育4 h。最后每孔加入150 μl二甲亚砜(DMSO),测定570 nm处吸光度。吸光度被用来衡量细胞活力。通过在对照培养基中培养的细胞吸光度归一化,认为其100%存活。 体外的抗病毒活性实验[3] 用SARS-CoV-2感染Vero-E6细胞(MOI = 0.05), 37℃孵育1 h后,用丹参素与Vero-E6细胞预孵育1 h。加入新鲜的丹参素培养基,24 h后采集150 μL上清检测,采用qRT-PCR法测定病毒拷贝数,评价丹参素抗SARS-CoV-2的能力。 将SARS-CoV-2 S与Danshensu/丹参素提前不同浓度混合,37℃作用30 min,并将100 μL混合物加入ACE2过表达的HEK-293T和Vero-E6细胞中。共孵育12 h后,丢弃上清,补充等量新鲜培养基。48 h后,用PBS洗涤细胞并裂解。检测细胞裂解液中的荧光素酶水平,以评估丹参素抗sars - cov - 2s的能力。 MTT试验[4] MTT法测定FaDu和Ca9-22细胞的活力。实验中,将细胞接种于24孔板上,用不同浓度的Danshensu/丹参素钠 (0-100 μM)处理24 h。处理后,采用MTT(3-(4,5-二甲基噻唑-2-基)-2,5-二苯四唑溴)测定细胞活力,如先前所定义。 创面愈合试验[4] 自插入时,将适量的FaDu和Ca9-22细胞接种到培养插入孔中孵育过夜。然后,在创面后分别用0、25、50和100 μM钠丹参素处理细胞0、3、6、8和24 h。然后拍摄细胞并测量平均爬行距离。 细胞系和程序[5] 人肾近端小管上皮细胞(HK-2细胞)来自美国ScienCell研究实验室。HK-2细胞在含1.0%青霉素-链霉素溶液和10%热灭活胎牛血清的DMEM培养基中,在5.0% CO2加湿培养箱中37℃培养。3-5代的细胞在恢复后用于整个研究。 实验中,HK-2细胞以104个/孔的密度接种于96孔板中。培养24 h后,细胞用丹参素(2.5-10µM)和顺铂处理18 h。然后收集各组细胞悬液进行活力、抗氧化、抗炎等分析。 |
| 动物实验 |
A total of 48 mice were used in this study and randomly separated into 6 groups (n = 8), including blank group (24 h and 72 h), VSV-G group (24 h and 72 h), SARS-CoV-2 S group (24 h and 72 h). ALI model was induced by SARS-CoV-2 S via trachea in SARS-CoV-2 S groups, while blank groups received DMEM via trachea and VSV-G groups received VSV-G via trachea.
Another total of 72 mice were used in this study and randomly separated into 9 groups (n = 8), including blank group, VSV-G group, SARS-CoV-2 S group, Danshensu 25 mg/kg group, Danshensu 50 mg/kg group, Danshensu 100 mg/kg group, Danshensu 25 mg/kg i.v. group, Danshensu 50 mg/kg i.v. group, Danshensu 100 mg/kg i.v. group. Blank group received distilled water orally, VSV-G group received distilled water orally, SARS-CoV-2 S group received distilled water orally. Danshensu i.v. groups received distilled water orally, and only received Danshensu (i.v. 25, 50, or 100 mg/kg, dissolved in normal saline for tail vein injection) once before the intratracheally received SARS-CoV-2 S at the 7th day; Danshensu groups received Danshensu (Oral administration. 25, 50, or 100 mg/kg/d, dissolved in distilled water) orally. All groups except Danshensu (i.v. 25, 50, or 100 mg/kg) were administrated once daily for 7 continuous days, Danshensu (i.v. 25, 50, or 100 mg/kg) groups just received distilled water orally for 7 continuous days before the tail vein injection. ALI model was induced by SARS-CoV-2 S via trachea after the last treatment, blank groups received DMEM via trachea and VSV-G groups received VSV-G via trachea. [3] After a 7-day period of acclimation to the experimental area, the mice were randomly assigned to 5 groups (n = 10/group) as follows: control group, cisplatin group (25 mg/kg), cisplatin (25 mg/kg) + Danshensu (15 mg/kg), cisplatin (25 mg/kg) + Danshensu (30 mg/kg) and cisplatin (25 mg/kg) + Danshensu (60 mg/kg). Before the in vivo experiments, all the mice were starved for 12 h. After fasting, all other groups except the control group were administered a single intraperitoneal injection of 25 mg/kg cisplatin. 1 h later,cisplatin (25 mg/kg) + Danshensu (15 mg/kg, 30 mg/kg, 60 mg/kg) groups were treated with corresponding doses of Danshensu once a day for 7 consecutive days. After the last of administration, blood samples were collected to prepare serum, the serum was stored at −80 °C in polystyrene tubes until analysis. Both kidneys were quickly dissected and weighed immediately following sacrifice. The kidneys and the serum were processed and stored for later various items analysis.[5] Assessment antioxidant activity of Danshensu [5] The active oxygen scavenging activity of Danshensu was determined as the protocol described below. The kidney tissues in each group were homogenized in ice-cold phosphate buffer saline (PBS) (0.05 M, PH 7) and centrifuged at 10,000g for 10 min at 4 ℃. The supernatants were collected for the estimation of malondialdehyde (MDA), reactive oxygen species (ROS) levels and superoxide dismutase (SOD), Glutathione peroxidase (GPx), Catalase (CAT) activities determined using commercial kits according to the manufacturer’s instructions. HK-2 cells were seeded at a density of 104 cells /well in 96-well plates. After 24 h of culture, the cells were treated with Danshensu and cisplatin for 18 h. Then, the cells were incubated with 2, 7-dichlorofluorescein diacetate (DCF-DA) and Hoechst33342 for 20 min the result was expressed as percentage change fluorescence, where the control group was taken as 100%. |
| 参考文献 |
|
| 其他信息 |
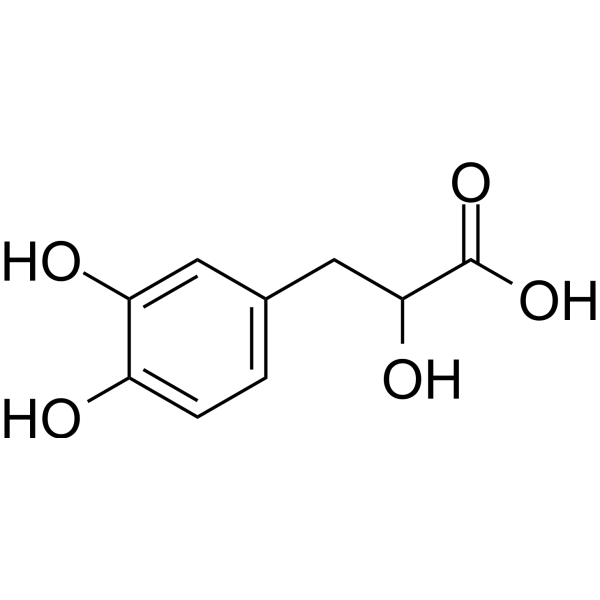
3-(3,4-dihydroxyphenyl)lactic acid is a 2-hydroxy monocarboxylic acid and a member of catechols. It is functionally related to a rac-lactic acid. It is a conjugate acid of a 3-(3,4-dihydroxyphenyl)lactate.
3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoic acid has been reported in Salvia miltiorrhiza, Salvia officinalis, and other organisms with data available. See also: 3-(3,4-Dihydroxyphenyl)lactate (annotation moved to). In conclusion, salvianolic acid B and Danshensu of caffeic acid derivatives isolated from S. miltiorrhiza were shown to be efficient antioxidants. Both salvianolic acid B and danshensu showed higher free radical scavenging activity than vitamin C, but lower iron chelating and hydrogen peroxide scavenging activities. Their antioxidant capacities are in agreement with their protective effects against cell injury from oxidative stresses. Condensation and conjugation of caffeic acid and its derivatives appears important for antioxidant activity. Furthermore, salvianolic B was a better antioxidant than danshensu. These results suggested that phenolic antioxidants isolated from natural resources could also have potential applications in food industry.[1] Danshen, the dried root of Salvia miltiorrhiza, has been widely used in China and, to a lesser extent, in Japan, the United States, and other European countries for the treatment of cardiovascular and cerebrovascular diseases. In China, the specific clinical use is angina pectoris, hyperlipidemia, and acute ischemic stroke. The current review covers its traditional uses, chemical constituents, pharmacological activities, pharmacokinetics, clinical applications, and potential herb-drug interactions based on information obtained in both the English and Chinese literature. Although numerous clinical trials have demonstrated that certain Danshen products in China are effective and safe for the treatment of cardiovascular diseases, most of these lack sufficient quality. Therefore, large randomized clinical trials and further scientific research to determine its mechanism of actions will be necessary to ensure the safety, effectiveness, and better understanding of its action.[2] Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can induce acute inflammatory response like acute lung inflammation (ALI) or acute respiratory distress syndrome, leading to severe progression and mortality. Therapeutics for treatment of SARS-CoV-2-triggered respiratory inflammation are urgent to be discovered. Our previous study shows that Salvianolic acid C potently inhibits SARS-CoV-2 infection. In this study, we investigated the antiviral effects of a Salvia miltiorrhiza compound, Danshensu, in vitro and in vivo, including the mechanism of S protein-mediated virus attachment and entry into target cells. In authentic and pseudo-typed virus assays in vitro, Danshensu displayed a potent antiviral activity against SARS-CoV-2 with EC50 of 0.97 μM, and potently inhibited the entry of SARS-CoV-2 S protein-pseudo-typed virus (SARS-CoV-2 S) into ACE2-overexpressed HEK-293T cells (IC50 = 0.31 μM) and Vero-E6 cell (IC50 = 4.97 μM). Therefore, we confirmed that Danshensu could induce the expression of antioxidant enzyme and suppress the production of inflammatory mediator as a beneficial anti-inflammatory agent in vivo. ALI in mice could be induced by SARS-CoV-2 S and reversed in biochemical and pathological indexes by Danshensu. Meanwhile, our results revealed that the model induced by SARS-CoV-2 S via trachea could be used for the experiment in vivo of anti-inflammatory drug for the inflammatory response of COVID-19.[3] In conclusion, the present study demonstrates the effect of sodium Danshensu, which is a bioactive, water soluble, phenolic compound found in Salvia miltiorrhiza (Danshen), on cancer cell migration and invasion in human OSCC. The study findings reveal that sodium danshensu significantly reduces the motility and metastatic ability of oral cancer cells by reducing the phosphorylation of p38 MAPK. However, sodium danshensu-mediated alteration in ERK1/2 phosphorylation was observed only in FaDu cells. Collectively, the present study identifies sodium danshensu as a potential natural anticancer agent that can be used therapeutically to manage highly metastatic OSCC. [4] Both inflammatory cytokines and NLRP3 inflammasome can be regulated by the NF-κB signaling pathway. Moreover, accumulating evidence suggests that there is crosstalk between the Nrf2 and the NF-ĸB signaling pathway. Some studies have observed that the activation of Nrf2 retarded the NF-ĸB-mediated proinflammatory reaction. Nrf2 knockout mouse showed increased activation of NF-ĸB. Nrf2 is an oxidative stress axis factor, which plays a key role in attenuating the oxidant-induced injury. While oxidative stress occurs, Nrf2 dissociated from Keap1, transferred from the cytoplasm to the nucleus and combined with antioxidant response elements to activate the antioxidant enzyme genes (such as HO-1, NQO1) transcription and expression. It is revealed in this study that Danshensu upregulated the expression of Nrf2 and HO-1 proteins and suppressed the phosphorylation and subsequent translocation of nuclear transcription factor-kappa B (NF-κB) to the nucleus, through the inhibition of the IκB kinase complex (IKK) activation and IκBα protein phosphorylation. Therefore, it can be inferred that Danshensu may alleviate oxidative stress and inflammation to exert a protective effect on cisplatin-induced nephrotoxicity by activating Nrf2 pathway and inhibiting NF-ĸB signaling pathway.[5] |
| 分子式 |
C9H10O5
|
|---|---|
| 分子量 |
198.172
|
| 精确质量 |
198.053
|
| 元素分析 |
C, 54.55; H, 5.09; O, 40.37
|
| CAS号 |
23028-17-3
|
| 相关CAS号 |
Danshensu sodium salt;67920-52-9
|
| PubChem CID |
439435
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.54g/cm3
|
| 沸点 |
480.3ºC at 760mmHg
|
| 闪点 |
258.4ºC
|
| LogP |
0.085
|
| tPSA |
97.99
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
205
|
| 定义原子立体中心数目 |
0
|
| SMILES |
OC(C(=O)O)CC1C=CC(=C(C=1)O)O
|
| InChi Key |
PAFLSMZLRSPALU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H10O5/c10-6-2-1-5(3-7(6)11)4-8(12)9(13)14/h1-3,8,10-12H,4H2,(H,13,14)
|
| 化学名 |
3-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid
|
| 别名 |
23028-17-3; 3-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid; 3,4-Dihydroxyphenyllactic acid; (Rac)-Salvianic acid A; 3-(3,4-dihydroxyphenyl)lactic acid; Benzenepropanoic acid, alpha,3,4-trihydroxy-; Sodium Danshensu; NA8H56YM3K;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.0462 mL | 25.2309 mL | 50.4617 mL | |
| 5 mM | 1.0092 mL | 5.0462 mL | 10.0923 mL | |
| 10 mM | 0.5046 mL | 2.5231 mL | 5.0462 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。