| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
Angiotensin II receptor
Angiotensin II type 1 receptor (AT1R) [1] - Toll-like receptor 2 (TLR2) [2] - Angiotensin II type 1 receptor (AT1R) [3] |
|---|---|
| 体外研究 (In Vitro) |
缬沙坦 (CGP 48933)(一种合成的非肽血管紧张素 II 1 型受体拮抗剂)通过抑制血管紧张素的作用来扩张血管并降低血压。当用缬沙坦治疗时,老化的主动脉内皮细胞表现出 AT1R 表达的大幅降低[1]。当缬沙坦预处理时,促炎细胞因子和 TLR2 信号传导受到抑制。饮酒后,AGTR1 的表达上调,缬沙坦预处理可阻断这种表达[2]。
- TLR2信号抑制:在人主动脉内皮细胞(HAECs)中,缬沙坦(10 μM)阻断酒精诱导的TLR2上调(TLR2蛋白表达减少60%)及后续NF-κB激活(p65磷酸化降低55%)。荧光素酶报告基因实验显示NF-κB驱动的荧光素酶活性降低70%[2] - COX2表达抑制:在高糖刺激的人系膜细胞(HMCs)中,缬沙坦(1.25–10 μM)剂量依赖性地使COX2蛋白水平较溶媒组降低30–60%(Western blot检测)。该效应独立于AT1R拮抗作用[4] 在从老年大鼠分离的原代主动脉平滑肌细胞(ASMCs)中,缬沙坦(Valsartan, CGP-48933) (1-10 μM)呈剂量依赖性抑制Ang II诱导的ERK磷酸化。10 μM时,磷酸化ERK(p-ERK)水平较Ang II处理组降低60%,基质金属蛋白酶-9(MMP-9)表达降低45%[1] - 在经酒精(100 mM)诱导炎症的人脐静脉内皮细胞(HUVECs)中,缬沙坦(Valsartan, CGP-48933) (10-50 μM)抑制TLR2信号通路。50 μM时,促炎细胞因子mRNA表达降低(IL-6降低55%,TNF-α降低48%),NF-κB核转位受抑(p65亚基核积累减少50%)[2] - 在从心梗(MI)后小鼠分离的心肌成纤维细胞中,缬沙坦(Valsartan, CGP-48933) (5-20 μM)调节TGF-β1和HIF-1α表达。20 μM时,TGF-β1蛋白水平降低40%,HIF-1α蛋白水平升高2.2倍,进而抑制胶原合成(胶原I降低35%,胶原III降低30%)[3] |
| 体内研究 (In Vivo) |
在患有 MI 的大鼠中,缬沙坦 (CGP 48933) 显着降低 TGF-β/Smad、Hif-1α 和纤维化相关蛋白的表达。缬沙坦与生理盐水和肼屈嗪相比,对缺血心脏的心功能、梗死面积、壁厚度和心肌血管化有相当大的改善[3]。高盐饮食可导致高血压、心脏损伤(如纤维化和炎症细胞浸润)、水通道蛋白 1 和血管生成因子的抑制,以及缬沙坦可以部分逆转的其他后果[4]。缬沙坦是一种有效的抗抑郁和抗焦虑药物,可以增加 BDNF 表达和海马神经发生。长期服用缬沙坦(5-40 mg/kg/d,口服)可减少 TST 和 FST 中的不动时间,延长 OFT 中的视野中心时间和 NSF 中的进食潜伏期,并增强对蔗糖的偏好在SPT[5]中。
- 衰老诱导的主动脉退化:在老年大鼠(24月龄)中,缬沙坦(10 mg/kg/天口服8周)使主动脉中膜厚度减少28%,弹性纤维断裂评分降低35%。这些效应与ERK1/2磷酸化减少(p-ERK1/2水平降低40%)和eNOS表达增加相关[1] - 心肌梗死模型:在心肌梗死后大鼠中,缬沙坦(20 mg/kg/天口服4周)减少心脏纤维化面积32%,改善射血分数18%。这与TGF-β1协同抑制(蛋白水平降低45%)和HIF-1α上调(mRNA增加2.3倍)相关[3] - 高盐饮食心脏保护:在高盐饮食(8% NaCl,4周)的C57BL/6小鼠中,缬沙坦(10 mg/kg/天口服)使心脏水通道蛋白1(AQP1)表达恢复正常(溶媒组降低50%),并使VEGF水平增加2.1倍,改善微血管密度[4] - 抑郁/焦虑样行为:在慢性轻度应激(CMS)小鼠中,缬沙坦(10 mg/kg/天腹腔注射4周)使强迫游泳实验不动时间减少40%,海马BDNF蛋白水平增加1.8倍。这些效应可被AT1R激动剂Ang II逆转[5] 在老年大鼠(24月龄)中,口服缬沙坦(Valsartan, CGP-48933) (30 mg/kg/天,持续8周)改善主动脉退化:主动脉弹性蛋白含量增加35%,主动脉壁厚度减少25%,ASMC凋亡数量减少(TUNEL阳性细胞:对照组22个/mm²,给药组8个/mm²);同时将收缩压(SBP)从165 mmHg降至130 mmHg[1] - 在心肌梗死(左前降支结扎诱导)小鼠中,口服缬沙坦(Valsartan, CGP-48933) (20 mg/kg/天,持续4周)发挥心脏保护作用:左心室舒张末期直径(LVEDD)减少20%,左心室射血分数(LVEF)增加18%,心肌胶原沉积减少(胶原体积分数:心梗对照组30%,给药组15%);此外,心肌组织中HIF-1α上调1.8倍,TGF-β1下调45%[3] - 在短期高盐饮食(8% NaCl,2周)小鼠中,口服缬沙坦(Valsartan, CGP-48933) (15 mg/kg/天,持续2周)保护心脏功能:恢复心脏水通道蛋白1(AQP1)表达(较盐负荷组增加1.2倍),血管内皮生长因子(VEGF)水平增加40%,阻止高盐诱导的心脏含水量升高(从79%降至75%)[4] - 在不可预测慢性轻度应激(UCMS,4周)小鼠中,口服缬沙坦(Valsartan, CGP-48933) (10 mg/kg/天,持续2周)逆转抑郁/焦虑样行为:强迫游泳实验中不动时间减少35%,旷场实验中中央区停留时间增加40%;同时诱导海马神经发生(BrdU阳性细胞:UCMS组20个/mm²,给药组45个/mm²),海马BDNF蛋白表达上调2.1倍[5] |
| 酶活实验 |
将主动脉组织或细胞样品在裂解缓冲液A(20 mM Tris-HCl,pH8.0,150 mM NaCl,1%Triton X-100,2 mM EDTA,1 mM苯基甲基磺酰氟,20μg/ml抑肽酶、10μg/ml亮蛋白肽、20 mMß-甘油酸盐和2 mM NaF)中均化30分钟。离心匀浆,并用BCA蛋白质测定试剂盒(Piece Biotech股份有限公司,Rockford,IL,USA)测定蛋白质浓度。将每个样品提取物中等量的蛋白质(大多数蛋白质为20μg/lane,而p-p38和p-JNK检测为100μg/lanne)加载在12.5%的SDS-PAGE凝胶中进行电泳,并电印迹到PDVF膜上。在室温下用5%脱脂奶粉(在TBST中)封闭膜2小时,然后与一级抗体在4°C下孵育过夜。然后,用TBST洗涤膜(10分钟×3),并与辣根过氧化物酶偶联的二级抗体在室温下孵育1小时(所有抗体均购自Cell Signaling Technology,Boston,MA,USA)。用TBST(10分钟×3)洗涤后,使用ECL蛋白质印迹检测系统(Amersham Pharmacia Biotech,Piscataway,NJ,USA)开发免疫印迹,并通过将免疫印迹暴露于X射线胶片进行记录[1]。
AT1R介导的ERK磷酸化实验:将原代大鼠ASMCs接种于6孔板,无血清培养24小时后,用缬沙坦(Valsartan, CGP-48933) (1-10 μM)预处理30分钟,再用Ang II(100 nM)刺激15分钟。裂解细胞后进行Western blot,检测p-ERK和总ERK,用图像分析软件定量条带强度,计算p-ERK抑制率[1] - TLR2活性实验:将HUVECs接种于6孔板,用酒精(100 mM)和缬沙坦(Valsartan, CGP-48933) (10-50 μM)处理24小时。提取核蛋白,用ELISA试剂盒检测NF-κB p65亚基核含量;提取总RNA,逆转录合成cDNA后进行实时荧光定量PCR,检测IL-6和TNF-α mRNA水平(以GAPDH为内参基因)[2] |
| 细胞实验 |
将主动脉切成小块,在4°C的0.2M二氧化二钙缓冲液(pH 7.4)中的2.5%戊二醛中固定2小时,然后在PBS中洗涤。将材料在2%OsO4溶液中孵育,在一系列增加的乙醇浓度和环氧丙烷中脱水,最后浸入Spurr树脂中。在安装在铜网格上的Leica ultracut UCT超微切片机(Leica Microsystems Inc,LKB-II,Wetzlar,Germany)上切割超薄切片(50 nm),并在JEM 1200EX透射电子显微镜下检查[1]。
- TLR2/NF-κB通路实验:HAECs经缬沙坦(1–10 μM)预处理1小时,再用酒精(50 mM)刺激24小时。通过Western blot检测TLR2和p-NF-κB p65水平。荧光素酶报告质粒(NF-κB响应型)在酒精暴露前24小时转染入细胞[2] - COX2表达实验:HMCs在高糖(30 mM)中与缬沙坦(1.25–10 μM)共培养48小时。提取总蛋白,通过Western blot定量COX2水平,以β-肌动蛋白作为内参[4] 主动脉平滑肌细胞(ASMC)凋亡实验:从老年大鼠分离原代ASMCs,接种于24孔板,用缬沙坦(Valsartan, CGP-48933) (1-10 μM)处理48小时。4%多聚甲醛固定细胞,0.1%曲拉通X-100透化后,加入TUNEL反应液37°C孵育60分钟。DAPI染核后,荧光显微镜下计数TUNEL阳性细胞(每孔5个视野)[1] - 心肌成纤维细胞胶原合成实验:从心梗后小鼠分离心肌成纤维细胞,接种于24孔板,用缬沙坦(Valsartan, CGP-48933) (5-20 μM)处理72小时。ELISA试剂盒检测细胞培养上清中胶原I和III水平;提取总蛋白后进行Western blot,检测TGF-β1和HIF-1α表达(以β-肌动蛋白为内参)[3] |
| 动物实验 |
Twenty young (or adult, 3-month-old) and 40 aged (18-month-old) male Wistar rats were purchased from the Department of Laboratory Animals, China Medical University. Animals were maintained at controlled temperature of 21°C and in a 12-hour day/night cycle. All the experimental procedures were approved by the Institutional Animal Care and Use Committee of China Medical University.
Young or adult animals were used as control group. Aged animals were randomly divided into two groups: the ageing group and Valsartan group (n = 20 in each group). The control and the ageing animals had free access to water and standard rat chow. The valsartan group animals continually took valsartan (Novartis Pharma Stein AG; 30 mg/kg/day) in their drinking water for 6 months. The concentration of valsartan dissolved in the drinking water was determined based on the previously established rats drinking patterns [1].
- Ageing Rat Model: Male Wistar rats (24 months) received Valsartan (10 mg/kg/day) or vehicle via oral gavage for 8 weeks. Aortic samples were collected for histology (Masson’s trichrome staining) and protein analysis (Western blot for p-ERK1/2 and eNOS) [1] - MI Rat Model: Sprague-Dawley rats underwent left coronary artery ligation. Starting 24 h post-MI, Valsartan (20 mg/kg/day) or vehicle was administered orally for 4 weeks. Cardiac fibrosis was assessed by picrosirius red staining, and TGF-β1/HIF-1α levels were measured by ELISA and qPCR, respectively [3] - CMS Mouse Model: C57BL/6 mice were exposed to CMS for 6 weeks. Valsartan (10 mg/kg/day) or vehicle was injected intraperitoneally during weeks 3–6. Hippocampal BDNF levels were measured by ELISA, and behavioral tests (forced swim test, sucrose preference test) were conducted weekly [5] Aged rat aortic degeneration model: Use male Sprague-Dawley rats (24 months old). Administer Valsartan (CGP-48933) (30 mg/kg/day) by oral gavage (dissolved in 0.5% carboxymethyl cellulose) for 8 weeks. The control group receives the same volume of vehicle. Measure SBP weekly using tail-cuff plethysmography. At the end of the experiment, harvest aortic tissues for elastin staining (Verhoeff-Van Gieson stain), histomorphometric analysis (wall thickness measurement), and TUNEL staining [1] - MI mouse cardiac protection model: Use male C57BL/6 mice (8-10 weeks old). Induce MI by ligating the left anterior descending coronary artery. One week after MI, administer Valsartan (CGP-48933) (20 mg/kg/day) by oral gavage (dissolved in 0.5% methylcellulose) for 4 weeks. Perform echocardiography before sacrifice to measure LVEDD and LVEF. Harvest hearts for Masson's trichrome staining (collagen deposition analysis) and Western blot (TGF-β1 and HIF-1α detection) [3] - High-salt diet mouse model: Use male C57BL/6 mice (6-8 weeks old). Feed mice an 8% NaCl high-salt diet and administer Valsartan (CGP-48933) (15 mg/kg/day) by oral gavage (dissolved in 0.5% carboxymethyl cellulose) for 2 weeks. The control group receives a normal diet and vehicle. After sacrifice, collect cardiac tissues to measure water content (dry-wet weight ratio), and detect AQP1 and VEGF expression by Western blot [4] - UCMS mouse depression model: Use male C57BL/6 mice (8-10 weeks old). Subject mice to UCMS (including food/water deprivation, cage tilting, cold stress, etc.) for 4 weeks. Then administer Valsartan (CGP-48933) (10 mg/kg/day) by oral gavage (dissolved in 0.5% methylcellulose) for 2 weeks. Perform forced swim test and open field test to evaluate behavior. Inject BrdU (50 mg/kg, intraperitoneal) twice weekly during drug administration, then harvest hippocampi for BrdU immunostaining (neurogenesis) and BDNF Western blot [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After one oral dose, the antihypertensive activity of valsartan begins within approximately 2 hours and peaks within 4-6 hours in most patients. Food decreases the exposure to orally administered valsartan by approximately 40% and peak plasma concentration by approximately 50%. AUC and Cmax values of valsartan generally increase linearly with increasing dose over the therapeutic dose range. Valsartan does not accumulate appreciably in plasma following repetitive administration. Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites. The steady-state volume of distribution of valsartan after intravenous administration is small (17 L), indicating that valsartan does not distribute into tissues extensively. Following intravenous administration, plasma clearance of valsartan is approximately 2 L/hour and its renal clearance is 0.62 L/hour (about 30% of total clearance). Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites. ... Following intravenous administration, plasma clearance of valsartan is about 2 L/hr and its renal clearance is 0.62 L/hr (about 30% of total clearance). Absolute bioavailability for the capsule formulation is approximately 25% (range, 10-35%). Food decreases the area under the plasma concentration-time curve (AUC) and peak plasma concentration by approximately 40 and 50%, respectively. Valsartan peak plasma concentration is reached 2 to 4 hours after dosing. Valsartan shows bi-exponential decay kinetics following intravenous administration, with an average elimination half-life of about 6 hours. Absolute bioavailability for Diovan is about 25% (range 10% to 35%). The bioavailability of the suspension is 1.6 times greater than with the tablet. With the tablet, food decreases the exposure (as measured by AUC) to valsartan by about 40% and peak plasma concentration (Cmax) by about 50%. AUC and Cmax values of valsartan increase approximately linearly with increasing dose over the clinical dosing range. Valsartan does not accumulate appreciably in plasma following repeated administration. The steady state volume of distribution of valsartan after intravenous administration is small (17 L), indicating that valsartan does not distribute into tissues extensively. Valsartan is highly bound to serum proteins (95%), mainly serum albumin. For more Absorption, Distribution and Excretion (Complete) data for VALSARTAN (6 total), please visit the HSDB record page. Metabolism / Metabolites Valsartan undergoes minimal liver metabolism and is not biotransformed to a high degree, as only approximately 20% of a single dose is recovered as metabolites. The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. In vitro metabolism studies involving recombinant CYP 450 enzymes indicated that the CYP 2C9 isoenzyme is responsible for the formation of valeryl-4-hydroxy valsartan. Valsartan does not inhibit CYP 450 isozymes at clinically relevant concentrations. CYP 450 mediated drug interaction between valsartan and coadministered drugs are unlikely because of the low extent of metabolism. Valsartan is known to be excreted largely as unchanged compound and is minimally metabolized in man. Although the only notable metabolite is 4-hydroxyvaleryl metabolite (4-OH valsartan), the responsible enzyme has not been clarified at present. The current in vitro studies were conducted to identify the cytochrome P450 (CYP) enzymes involved in the formation of 4-OH valsartan. Valsartan was metabolized to 4-OH valsartan by human liver microsomes and the Eadie-Hofstee plots were linear. The apparent Km and Vmax values for the formation of 4-OH valsartan were 41.9-55.8 microM and 27.2-216.9 pmol min(-1) mg(-1) protein, respectively. There was good correlation between the formation rates of 4-OH valsartan and diclofenac 4'-hydroxylase activities (representative CYP2C9 activity) of 11 individual microsomes (r = 0.889). No good correlation was observed between any of the other CYP enzyme marker activities (CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and CYP4A). Among the recombinant CYP enzymes examined (CYPs 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4, 3A5 and 4A11), CYP2C9 notably catalysed 4-hydroxylation of valsartan. For the specific CYP inhibitors or substrates examined (furafylline, diclofenac, S(+)-mephenytoin, quinidine and troleandomycin), only diclofenac inhibited the formation of 4-OH valsartan. These results showed that CYP2C9 is the only form responsible for 4-hydroxylation of valsartan in human liver microsomes. Although CYP2C9 is involved in valsartan metabolism, CYP-mediated drug-drug interaction between valsartan and other co-administered drugs would be negligible. ... Valsartan, when administered as an oral solution, is primarily recovered in feces (about 83% of dose) and urine (about 13% of dose). The recovery is mainly as unchanged drug, with only about 20% of dose recovered as metabolites. The primary metabolite, accounting for about 9% of dose, is valeryl 4-hydroxy valsartan. In vitro metabolism studies involving recombinant CYP 450 enzymes indicated that the CYP 2C9 isoenzyme is responsible for the formation of valeryl-4-hydroxy valsartan. Valsartan does not inhibit CYP 450 isozymes at clinically relevant concentrations. CYP 450 mediated drug interaction between valsartan and coadministered drugs are unlikely because of the low extent of metabolism. ... Valsartan has known human metabolites that include 4-hydroxy-valsartan. Biological Half-Life After intravenous (IV) administration, valsartan demonstrates bi-exponential decay kinetics, with an average elimination half-life of about 6 hours. Valsartan shows bi-exponential decay kinetics following intravenous administration, with an average elimination half-life of about 6 hours. ... In an investigation of pharmacokinetics and pharmacodynamics in normotensive male volunteers, valsartan was rapidly absorbed with the maximal plasma concentration occurring 2-3 hr after oral administration. The elimination half-life was about 4-6 hr, valsartan was poorly metabolized, and most of the drug was excreted via feces. ... - Oral Absorption: In rats, Valsartan (10 mg/kg orally) achieved a Cmax of 280 ng/mL at Tmax of 2 h. Absolute bioavailability was 23%, consistent with human data [1] - Plasma Protein Binding: Valsartan showed >95% plasma protein binding in rat and human plasma, primarily to albumin [1] - Metabolism: Valsartan undergoes minimal hepatic metabolism, with <10% of the dose converted to inactive metabolites. The parent drug is excreted 83% via bile and 17% via urine [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Valsartan is a white to practically white fine powder that is formulated into oral tablets. Valsartan is an angiotensin II type 1 (AT1) receptor antagonist. It is used in the management of hypertension. Valsartan is also used to treat heart failure or left ventricular dysfunction after acute myocardial infarction. HUMAN EXPOSURE AND TOXICITY: The most likely manifestations of overdosage include hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. Depressed levels of consciousness, circulatory collapse and shock have been reported. The use of valsartan during pregnancy is contraindicated. While use during the first trimester does not suggest a risk of major anomalies, use during the second and third trimester may cause teratogenicity and severe fetal and neonatal toxicity. Fetal toxic effects may include anuria, oligohydramnios, fetal hypocalvaria, intrauterine growth restriction, prematurity, and patent ductus arteriosus. Anuria-associated anhydramnios/oligohydramnios may produce fetal limb contractures, craniofacial deformation, and pulmonary hypoplasia. Severe anuria and hypotension, resistant to both pressor agents and volume expansion, may occur in the newborn following in utero exposure to valsartan. ANIMAL STUDIES: There was no evidence of carcinogenicity when valsartan was administered in the diet to mice and rats for up to two years. Also, valsartan had no adverse effects on fertility of male or female rats and no teratogenic effects were observed when valsartan was administered to pregnant mice and rats. However, significant decreases in fetal weight, pup birth weight, pup survival rate, and slight delays in developmental milestones were observed in studies in which parental rats were treated with valsartan at oral, maternally toxic (reduction in body weight gain and food consumption) doses during organogenesis or late gestation and lactation. In rabbits, maternal toxic doses resulted in fetal resorptions, litter loss, abortions, and low fetal body weight as well as maternal mortality. Mutagenicity assays did not reveal any valsartan-related effects at either the gene or chromosome level. These assays included bacterial mutagenicity tests with Salmonella (Ames) and E coli, a gene mutation test with Chinese hamster V79 cells, a cytogenetic test with Chinese hamster ovary cells, and a rat micronucleus test. Hepatotoxicity Valsartan has been associated with a low rate of serum aminotransferase elevations ( Likelihood score: D (Possible rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Milk levels after the lowest dose of the combination of valsartan and sacubitril (Entresto) are very low. If the highest recommended maternal dosage (6 times greater) produces proportionate milk levels, they would likely still be quite low. Valsartan is unlikely to affect the nursing infant. ◉ Effects in Breastfed Infants Two women taking sacubitril 24 mg and valsartan 26 mg (Entresto) did not observe any symptoms in their breastfed infants. Their extent of breastfeeding was not reported. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Valsartan is highly bound to serum proteins (95%), mainly serum albumin. Interactions Concurrent use of valsartan and warfarin did not affect the pharmacokinetics of valsartan or the anticoagulant effect of warfarin. Concomitant use of potassium-sparing diuretics (e.g., amiloride, spironolactone, triamterene), potassium supplements, or potassium-containing salt substitutes with valsartan may result in increased hyperkalemic effects and, in patients with heart failure, increases in serum creatinine concentration. Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor antagonists, including Diovan. Monitor serum lithium levels during concomitant use. Dual Blockade of the Renin-Angiotensin System (RAS): Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on Diovan and other agents that affect the RAS. For more Interactions (Complete) data for VALSARTAN (11 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Marmoset gavage >1000 mg/kg (approximate) LD50 Rat gavage >2000 mg/kg (approximate) |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Angiotensin II Type 1 Receptor Blockers; Antihypertensive Agents Diovan is an angiotensin II receptor blocker (ARB) indicated for: treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. /Included in US product labeling/ Diovan is an angiotensin II receptor blocker (ARB) indicated for: reduction of cardiovascular mortality in clinically stable patients with left ventricular failure or left ventricular dysfunction following myocardial infarction. /Included in US product labeling/ Diovan is an angiotensin II receptor blocker (ARB) indicated for: treatment of heart failure (NYHA class II-IV); Diovan significantly reduced hospitalization for heart failure. /Included in US product labeling/ For more Therapeutic Uses (Complete) data for VALSARTAN (6 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Diovan as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Diovan as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimesters of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Diovan, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Diovan for hypotension, oliguria, and hyperkalemia. Angiotensin II (A-II) is the main effector of the renin-angiotensin system. A-II functions by binding its type 1 (AT1) receptors to cause vasoconstriction and retention of sodium and fluid. Several AT1 receptor antagonists-a group of drugs collectively called \"sartans\"-have been marketed during the past few years for treatment of hypertension and heart failure. At least 15 case reports describe oligohydramnios, fetal growth retardation, pulmonary hypoplasia, limb contractures, and calvarial hypoplasia in various combinations in association with maternal losartan, candesartan, valsartan, or telmisartan treatment during the second or third trimester of pregnancy. Stillbirth or neonatal death is frequent in these reports, and surviving infants may exhibit renal damage. The fetal abnormalities, which are strikingly similar to those produced by maternal treatment with angiotensin-converting enzyme (ACE) inhibitors during the second and third trimesters of pregnancy, are probably related to extreme sensitivity of the fetus to the hypotensive action of these drugs. ... Valsartan is distributed into milk in rats. It is not known whether valsartan is distributed into human milk. Discontinue nursing or the drug because of potential risk in nursing infants. For more Drug Warnings (Complete) data for VALSARTAN (21 total), please visit the HSDB record page. Pharmacodynamics Valsartan inhibits the pressor effects of angiotensin II with oral doses of 80 mg inhibiting the pressor effect by about 80% at peak with approximately 30% inhibition persisting for 24 hours. Removal of the negative feedback of angiotensin II causes a 2- to 3-fold rise in plasma renin and consequent rise in angiotensin II plasma concentration in hypertensive patients. Minimal decreases in plasma aldosterone were observed after administration of valsartan. In multiple-dose studies in hypertensive patients, valsartan had no notable effects on total cholesterol, fasting triglycerides, fasting serum glucose, or uric acid. Hypotension Excessive hypotension was rarely seen (0.1%) in patients with uncomplicated hypertension treated with valsartan alone. In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients receiving high doses of diuretics, symptomatic hypotension may occur. This condition should be corrected prior to administration of valsartan, or the treatment should start under close medical supervision. Caution should be observed when initiating therapy in patients with heart failure. Patients with heart failure given valsartan commonly have some reduction in blood pressure, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed. In controlled trials in heart failure patients, the incidence of hypotension in valsartan-treated patients was 5.5% compared to 1.8% in placebo-treated patients. If excessive hypotension occurs, the patient should be placed in the supine position and, if necessary, given an intravenous infusion of normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized. Impaired Renal Function Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on valsartan. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on valsartan. Hyperkalemia Some patients with heart failure have developed increases in potassium. These effects are usually minor and transient, and they are more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of valsartan may be required. - Mechanism of Action: Valsartan competitively antagonizes AT1R, blocking Ang II-mediated vasoconstriction, fibrosis, and inflammation. It also modulates non-AT1R pathways, such as suppressing TLR2/NF-κB signaling and enhancing BDNF expression [2,5] - Therapeutic Applications: Approved for hypertension, heart failure, and post-MI cardiac remodeling. Emerging evidence supports its use in diabetic nephropathy and neuropsychiatric disorders [1,5] - Clinical Efficacy: In the VALIANT trial (n=14,703), Valsartan reduced cardiovascular mortality in post-MI patients to a similar extent as captopril [10] - Safety Profile: Well-tolerated with low incidence of adverse effects. Common side effects include hypotension (5–8%) and hyperkalemia (2–3%) [1] Valsartan (CGP-48933) ameliorates age-related aortic degeneration by blocking AT1R-mediated ERK activation, which reduces ASMC apoptosis and MMP-9 expression, thereby preserving aortic elastin and structure [1] - The mechanism by which Valsartan (CGP-48933) inhibits alcohol-induced endothelial inflammation involves suppressing TLR2/NF-κB signaling, which decreases the production of pro-inflammatory cytokines [2] - In post-MI mice, Valsartan (CGP-48933) exerts cardiac protection through a synergistic mechanism: it downregulates TGF-β1 to reduce collagen synthesis and upregulates HIF-1α to promote angiogenesis, collectively improving left ventricular function [3] - Valsartan (CGP-48933) protects the heart from high-salt damage by normalizing cardiac AQP1 expression (to reduce water accumulation) and increasing VEGF (to enhance vascular function) [4] - Valsartan (CGP-48933) reverses UCMS-induced depressive behavior by promoting hippocampal neurogenesis and upregulating BDNF, which may be related to its modulation of the renin-angiotensin system in the brain [5] |
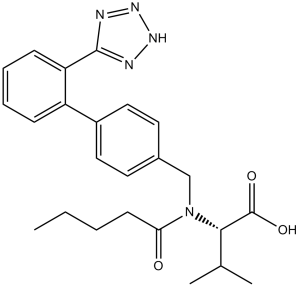
| 分子式 |
C24H29N5O3
|
|
|---|---|---|
| 分子量 |
435.52
|
|
| 精确质量 |
435.227
|
|
| 元素分析 |
C, 66.19; H, 6.71; N, 16.08; O, 11.02
|
|
| CAS号 |
137862-53-4
|
|
| 相关CAS号 |
Sacubitril/Valsartan;936623-90-4;Valsartan-d9;1089736-73-1;Valsartan-d3;1331908-02-1;Valsartan-d8;1089736-72-0;(Rac)-Valsartan-d9; 137862-53-4; 149690-05-1 (sodium)
|
|
| PubChem CID |
60846
|
|
| 外观&性状 |
White to off-white solid
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
684.9±65.0 °C at 760 mmHg
|
|
| 熔点 |
116-117°C
|
|
| 闪点 |
368.0±34.3 °C
|
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
|
| 折射率 |
1.587
|
|
| LogP |
4.75
|
|
| tPSA |
112.07
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
32
|
|
| 分子复杂度/Complexity |
608
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
O([H])C([C@]([H])(C([H])(C([H])([H])[H])C([H])([H])[H])N(C(C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H])=O)C([H])([H])C1C([H])=C([H])C(C2=C([H])C([H])=C([H])C([H])=C2C2N=NN([H])N=2)=C([H])C=1[H])=O
|
|
| InChi Key |
ACWBQPMHZXGDFX-QFIPXVFZSA-N
|
|
| InChi Code |
InChI=1S/C24H29N5O3/c1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23/h6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H,31,32)(H,25,26,27,28)/t22-/m0/s1
|
|
| 化学名 |
(S)-3-methyl-2-(N-{[2-(2H-1,2,3,4-tetrazol-5-yl)biphenyl-4-yl]methyl}pentanamido)butanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.74 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.74 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 10 mg/mL (22.96 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2961 mL | 11.4805 mL | 22.9611 mL | |
| 5 mM | 0.4592 mL | 2.2961 mL | 4.5922 mL | |
| 10 mM | 0.2296 mL | 1.1481 mL | 2.2961 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Changes in NT-proBNP, Safety, and Tolerability in HFpEF Patients With a WHF Event (HFpEF Decompensation) Who Have Been Stabilized and Initiated at the Time of or Within 30 Days Post-decompensation (PARAGLIDE-HF)
CTID: NCT03988634
Phase: Phase 3 Status: Completed
Date: 2024-07-29