| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
| 靶点 |
D3 dopamine receptor
|
|---|---|
| 体外研究 (In Vitro) |
多巴胺D3受体基因由Sokoloff及其同事于1990年鉴定。这一发现迅速引起了科学界的兴趣,因为这种意想不到的多巴胺受体亚型可能在抗精神病药物的抗精神病活性中发挥重要作用。它以高亲和力识别大多数神经抑制剂,其大脑分布主要局限于纹状体复合体的腹侧部分。然而,D3受体功能的表征和随后的鉴定最初受到至少四个重要因素的阻碍,这些因素仍部分未得到解决:(1)缺乏可以在体内区分D2和D3受体亚型功能的选择性药物,(2)缺乏与GTP依赖性蛋白的表观偶联,(3)对第二信使系统没有影响,以及(4)该受体在脑组织中的低表达水平;这些因素有助于缓和科学家的兴趣。然而,随着[3H]7-羟基-N,N-(二正丙基)-2-氨基四氢萘([3H]7-OH-DPAT)的鉴定,这种情况已经开始发生变化,[3H]7-1是多巴胺D3受体的第一个选择性配体。尽管其对D3与D2受体的结合选择性有点人为,但鉴定D3受体功能的潜在重要影响鼓励科学家将这种氨基四氢萘化合物用于体内研究,但成功有限。本评论主要关注7-OH-DPAT及其同系物的使用所产生的影响和争议,这项研究可能产生的新概念观点,以及部分选择性D3受体配体可能告诉我们的多巴胺D3受体功能。[2]
我们已经确定7-[3H]羟基-N,N-二正丙基-2-氨基四氢萘([3H]7-OH-DPAT)是最近克隆的多巴胺D3受体的选择性探针,并用它来评估这种受体的存在,并确定其在大脑中的分布和性质。在转染的中国仓鼠卵巢(CHO)细胞中,它以亚纳米亲和力结合D3受体,而在D2、D4和D1受体上,其亲和力分别低约100、1000和10000倍。在大鼠脑中鉴定出特定的[3H]7-OH-DPAT结合位点,Kd为0.8 nM,药理学与CHO细胞的参考D3受体相似。D3受体与D2受体在大脑中的不同之处在于其较低的丰度(2个数量级)和分布,仅限于少数主要系统发育古老的区域,如古纹状体和小脑弓,如膜结合放射自显影研究所证明的那样。大脑中的天然D3受体的特征是对多巴胺具有异常高的纳摩尔亲和力,鸟苷酸对激动剂结合的调节作用较低。这些不同的特征表明,D3受体参与了多巴胺神经元有限亚群中的一种特殊神经传递模式[3]。 |
| 体内研究 (In Vivo) |
假定的D-3多巴胺受体激动剂7-OH-DPAT(10微克/千克,皮下注射)减少了大鼠的自发活动,但没有诱导打哈欠;较高剂量(0.1-10.0 mg/kg,皮下注射)刺激了非定型的嗅闻、运动和咀嚼,选择性D-1拮抗剂BW 737C(5.0 mg/kg,皮下接种)减弱了这些刺激,但没有释放任何非典型行为。低剂量的7-OH-DPAT可能作用于抑制性D-3受体,而高剂量可能作用于刺激性D-3或其他参与与D-1受体合作但非对抗性相互作用的“D-2样”受体[1]。
全身施用D2样多巴胺能受体激动剂会增加打哈欠行为。然而,在患有病理状况的动物身上进行的研究很少。taiep大鼠是一种髓鞘突变体,最初髓鞘形成不足,随后进行性脱髓鞘,脑干是受影响最严重的区域之一。在我们的实验中,我们分析了系统性施用D2家族激动剂和拮抗剂对打哈欠行为的影响,并将其与8个月大的雄性taiep和Sprague-Dawley大鼠脑干和中枢神经系统(CNS)其他区域的脂质髓鞘含量相关联。受试者在标准条件下被置于有机玻璃笼中,光暗周期为12:12,灯在0700打开,可以自由接触啮齿动物颗粒和自来水。药物在0800时通过ip注射新鲜制备,观察受试者60分钟。使用拮抗剂时,在激动剂前15分钟给药。Sprague-Dawley和taiep大鼠在全身注射(-)-盐酸奎尼罗、R(+)-7-羟基-2-(二丙氨基)氢溴酸四氢萘(7-OH-DPAT)或反式-(±)-3,4,4a,10b-四氢-4-丙基-2H,5H-[1]苯并吡喃[4,3-b]-1,4-恶嗪-9-醇盐酸盐((±)-PD 128907)后,其打哈欠频率显著增加。在使用的D2样激动剂中,(-)-奎尼罗的效果更高。(-)舒必利可以减轻(-)-奎尼罗引起的影响;泰必利仅能减少由7-OH-DPAT引起的大鼠打哈欠。在Sprague-Dawley,只有(-)-舒必利能够减少(-)-quinpirole引起的打哈欠。总之,尽管taiep大鼠的髓鞘严重丧失,多巴胺能D2样激动剂仍然能够引起打哈欠。同样,患有影响髓鞘的各种中枢神经系统疾病的患者,如中风或多发性硬化症,能够打哈欠,这表明触发神经元仍然能够控制这种先天行为[5]。 多巴胺D3受体与精神分裂症的病理生理底物有关,主要拮抗D2受体的抗精神病药物在这种疾病中具有治疗活性。在本研究中,用神经抑制剂氟哌啶醇预处理了受7-OH-DPAT(7OH,一种选择性D3激动剂)诱导的运动减退的大鼠。与单独接受7OH的大鼠相比,这些动物表现出减弱的激动剂诱导的行为抑制。该药物组合还使额叶皮层的多巴胺代谢“正常化”,因为当同时给予7OH时,通常会通过急性抗精神病药物增强的周转率不再显著增加。这些观察表明,氟哌啶醇在调节边缘运动系统的皮质区域的作用可能对D3底物产生的精神分裂症症状的治疗效果很重要[6]。 |
| 动物实验 |
The compounds used in these studies were the D2-like agonists (−)-quinpirole hydrochloride, R(+)-7-Hydroxy-2-(dipropylamino)tetralin hydrobromide (7-OH-DPAT), or trans-(±)-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano [4,3-b]-1,4-oxazin-9-ol hydrochloride ((±)-PD 128,907), and the D2 antagonists were (−)-sulpiride hydrochloride, 5,6-dimethoxy-2-(di-n-propylamino)indan maleate (U-99194), and tiapride hydrochloride. All drugs were dissolved in sterile water and were freshly prepared at the beginning of each experimental session and administered by intraperitoneal injection (ip). The injection volume for all drugs was adjusted to 1 mL/kg. Sterile water served as the control injection. [5]
|
| 参考文献 |
[1]. Behavioural effects of the D3 DA receptor agonist 7-OH-DPAT in relation to other D2-like agonists. Neuropharmacology. 1993 May;32(5):509-10. [2]. Aminotetralin drugs and D3 receptor fuctions. Biochem Pharmacol. 1996 Aug 23;52(4):511-8. [3]. Identification, characterization, and localization of the DA D3 receptor in rat brain using [3H]-7-hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8155-9. [4]. DA receptor pharmacology. Trends Pharmacol Sci. 1994 Jul;15(7):264-70. |
| 其他信息 |
The dopamine exerts a tonic inhibitory control over cholinergic neurons that produced yawning (Holmgren and Urbá-Holmgren, 1980, Holmgren et al., 1982, Yamada and Furukawa, 1981). It is clearly established that an inverted U-shape dose–response curve was obtained with the ascending limb of the curve mediated by D3 receptors and the descending limb mediated by the D2 receptors (Collins et al., 2005, Collins et al., 2007, Baladi et al., 2010, Baladi et al., 2011). Because of the higher doses used in this study, only the descending limb of the curve was explored because at these doses there was a significant increase in the gripping-produced tonic-immobility episodes, a cardinal sign of this myelin-mutant rat (Eguibar et al., 2010), suggesting that taiep rats are less sensitive to D3 and D2 effects on yawning. For the D2-family antagonist used, the lack of effect is probably caused by the low spontaneous yawning frequency. However, tiapride is able to antagonize the increase of yawning frequency produced by 7-OH-DPAT in taiep rats because the myelin mutants had different sensitivity in D3 receptors and so this dose in Sprague–Dawley rats is on the descending limb of the dose–response curve, as previously suggested (Baladi et al., 2010, Baladi et al., 2011, Collins et al., 2007, Collins et al., 2009). Under free access to chow taiep and Sprague–Dawley rats showed different sensitivities to the action of the D2-like dopaminergic agonists, which could explain the differences obtained when antagonists were administered before the agonist, because at that dose the 7-OH-DPAT falls on the ascending limb of the dose–effect curve on taiep rats, but it could be on the descending limb in the Sprague–Dawley rats. The agonist is acting at D3 receptors in the former and quite probably in the D2 receptors in the latter (see Table 1 and Fig. 2). These differences in response to dopaminergic drugs can be because of age, gender, genetics, and nutritional status, as previously suggested (Baladi et al., 2011, Sevak et al., 2008). In our results it is quite clear that the differences in the response to tiapride and 7-OH-DPAT are caused by the genetic background of taiep rats and why yawning frequency differs in both groups of rats. [5]
Dopamine receptors are the primary targets in the treatment of schizophrenia, Parkinson's disease, and Huntington's chorea, and are discussed in this review by Philip Seeman and Hubert Van Tol. Improved therapy may be obtained by drugs that selectively target a particular subtype of dopamine receptor. Most antipsychotic drugs block D2 receptors in direct correlation to clinical potency, except clozapine, which prefers D4 receptors. D1 and D2 receptors can enhance each other's actions, possibly through subunits of the G proteins. In schizophrenia, the D2 and D3 receptor density is elevated by 10%, while the D4 receptor density is elevated by 600%. Therefore, D4 receptors may be a target for future antipsychotic drugs. While antipsychotics originally helped to discover dopamine receptors, the five cloned dopamine receptors are now facilitating the discovery of selective antipsychotic and antiparkinson drugs.[4] |
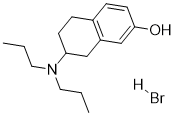
| 分子式 |
C16H26BRNO
|
|---|---|
| 分子量 |
328.28774
|
| 精确质量 |
327.12
|
| 元素分析 |
C, 58.54; H, 7.98; Br, 24.34; N, 4.27; O, 4.87
|
| CAS号 |
76135-30-3
|
| PubChem CID |
11957566
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
4.329
|
| tPSA |
23.47
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
237
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CCCN(CCC)C1CCC2=CC=C(C=C2C1)O.Br
|
| InChi Key |
ODNDMTWHRYECKX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H25NO.BrH/c1-3-9-17(10-4-2)15-7-5-13-6-8-16(18)12-14(13)11-15/h6,8,12,15,18H,3-5,7,9-11H2,1-2H31H
|
| 化学名 |
7-Hydroxy-N,N-dipropyl-2-aminotetralin hydrobromide
|
| 别名 |
7-OH DPAT; 7-OH-DPAT HBr; 159795-63-8; (A+-)-7-Hydroxy-2-(di-n-propylamino)tetralin hydrobromide; 633-919-6; 76135-30-3; 7-Hydroxy-DPAT hydrobromide; 7-(Dipropylamino)-5,6,7,8-tetrahydronaphthalen-2-ol hydrobromide; 7-OH-dpat hydrobromide; 2-Naphthalenol, 7-(dipropylamino)-5,6,7,8-tetrahydro-, hydrobromide; 7-OH-DPAT Hydrobromide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0461 mL | 15.2304 mL | 30.4609 mL | |
| 5 mM | 0.6092 mL | 3.0461 mL | 6.0922 mL | |
| 10 mM | 0.3046 mL | 1.5230 mL | 3.0461 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。