| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| Other Sizes |
|
| 靶点 |
Mitochondrial toxin; mitochondrial adenine nucleotide translocase (ANT)
|
|---|---|
| 体外研究 (In Vitro) |
Bongkrekic acid(0-50 μM;48 小时)的 EC50 分别为 34.14 μM、>50 μM 和 2.58 μM,可诱导 MDA-MB-231、MCF-7 和 LTED 细胞产生甲臜。在 LTED 细胞和亲代 MCF-7 细胞中,bongkrekic Acid(0.1–25 μM;48 小时)以剂量依赖性方式减少活细胞数量 [1]。
米酵菌酸/BKA/Bongkrekic acid对ERα阳性(MCF-7和LTED细胞)及ERα阴性MDA-MB-231细胞中甲臜生成的影响。[3] 我们首先使用MTS试剂[3-(4,5-二甲基噻唑-2-基)-5-(3-羧甲氧基苯基)-2-(4-磺苯基)-2H-四唑;Owen试剂]进行MTS实验,分析米酵菌酸/BKA(图1)和脂肪酸(FAs)是否影响乳腺癌细胞中甲臜的形成。MTS实验利用还原当量(如辅酶还原型烟酰胺腺嘌呤二核苷酸磷酸NADH)将MTS转化为有色甲臜产物;MTS可能被线粒体琥珀酸脱氢酶(复合体II组分)选择性裂解(图2)。该实验通常作为[3H]胸苷掺入法的替代方法。如图3所示,我们研究了BKA对三种乳腺癌细胞系(MDA-MB-231、MCF-7和LTED)甲臜生成的影响。虽然BKA在MCF-7细胞中显著促进甲臜形成(EC50=34.14 μM),但在MDA-MB-231细胞中仅微弱刺激(EC50>50 μM)(图3A)。当比较MCF-7与LTED细胞时,即使接种细胞数相同(5×103细胞/孔),LTED细胞的基线甲臜生成量仍比亲代MCF-7细胞高约1.5倍(图3B左图)。在LTED细胞实验中,BKA以EC50=2.58 μM有效刺激甲臜生成(图3B右图)。因此,BKA似乎优先加速乳腺癌细胞(尤其是ERα阳性的LTED细胞)的甲臜形成。 BKA/米酵菌酸介导的活细胞数量减少:LTED细胞与亲代MCF-7细胞的比较。[3] 甲臜生成的增强通常反映活细胞数量(即细胞活力)。若BKA处理的LTED细胞符合此规律,其他活细胞检测方法的结果应与MTS实验一致。我们使用钙黄绿素(calcein AM)探针和台盼蓝染色检测活细胞。该探针可穿透"活细胞"膜,被胞内酯酶水解为绿色荧光钙黄绿素。由于死细胞缺乏酯酶,活细胞被选择性标记。尽管25 μM BKA对MDA-MB-231细胞活力无影响(数据未显示),但在≤25 μM浓度下轻微减少MCF-7活细胞数量(图4A),并以浓度依赖性方式显著减少LTED活细胞(图4B)。台盼蓝排斥实验也检测到BKA介导的细胞生长抑制(数据未显示)。此外,与溶剂对照组相比,BKA更显著影响LTED细胞形态(暗示细胞死亡响应)(图4C)。EthD-III探针分析表明,BKA介导的细胞死亡不依赖坏死性凋亡(图4D)。 乳腺癌细胞中LDH-A的表达状态及米酵菌酸/BKA对其表达的影响。[3] LDH-A是乳腺组织中调节糖酵解过程丙酮酸转化为乳酸的主要亚型。侵袭性强的MDA-MB-231细胞即便在氧气存在下也通过糖酵解途径获取ATP(即"糖酵解"表型),将葡萄糖转化为乳酸并降低OXPHOS活性(图5A)。相比之下,非侵袭性/低致瘤性MCF-7细胞利用OXPHOS途径产ATP且糖酵解活性较低(即"氧化"表型)(图5B)。半定量和实时RT-PCR分析显示,LDH-A表达水平依次为:MDA-MB-231细胞(5.79/2.3倍)>> MCF-7细胞(1.0倍)=LTED细胞(0.76/1.3倍)(图6A)。因此LTED细胞与亲代MCF-7同属"氧化表型"。BKA和FAs(PA和AA)处理显示:AA对所有乳腺癌细胞无调节作用;PA普遍上调LDH-A表达;而BKA在LTED细胞中显著下调其表达(0.66倍 vs 对照1.0倍)(图6B-D)。 乳腺癌细胞中PDK4的表达状态及米酵菌酸/BKA对其表达的影响。[3] PDK4通过抑制线粒体丙酮酸脱氢酶复合体(PDH)调控葡萄糖氧化。实时RT-PCR显示三种乳腺癌细胞的基础PDK4表达差异显著:MDA-MB-231细胞(2.18倍)> MCF-7细胞(1.0倍)> LTED细胞(0.08倍)(图7A),表明LTED细胞生长高度依赖葡萄糖氧化供能。FAs处理中,两种FA均显著刺激MDA-MB-231细胞的PDK4表达;AA上调而PA下调MCF-7/LTED细胞的PDK4(图7B-D)。值得注意的是,BKA"选择性"下调LTED细胞的PDK4表达(图7D),与图6D中LDH-A的结果共同提示:BKA通过降低PDK4/LDH-A水平解除对PDH的抑制,从而促进葡萄糖利用。 乳腺癌细胞中Topo IIα的表达状态及米酵菌酸/BKA对其表达的影响。[3] 增殖标志物Topo IIα的表达强度依次为:MDA-MB-231细胞(1.3倍)> MCF-7细胞(1.0倍)> LTED细胞(0.54倍)(图8A)。25 μM PA降低MDA-MB-231细胞活力(图8B)。BKA仅下调LTED细胞的Topo IIα表达(图8D),此选择性调节与PDK4结果一致(见图6-7)。Ki-67检测进一步证实BKA对LTED细胞增殖的选择性抑制(0.41±0.014 vs 对照1.0, p<0.05)。与BKA促进甲臜形成相反(图2B),其降低了LTED细胞中增殖标志物(Topo IIα和Ki-67)水平和活细胞数量(图3B和8D)。 简化米酵菌酸/BKA类似物对甲臜生成及PPARγ介导转录活性的影响。[3] 我们合成两种简化BKA类似物BKA-1'和BKA-4,与BKA、BKA-2、BKA-3共同进行实验。其中仅BKA-3在50 μM时表现出弱于母体BKA的刺激活性(图3B和9B)。PPARγ转录实验显示BKA-3(4.86倍)、BKA-4(4.18倍)和BKA(2.07倍)激活PPARγ(图9C)。尽管BKA-3/BKA-4的PPARγ激活潜力约为BKA的2倍,但它们对甲臜形成的影响极弱或呈阴性,提示BKA对PPARγ的激活作用不直接参与LTED细胞死亡。 BKA/米酵菌酸对Cu2+介导氧化的不敏感性。[3] BKA结构中的二烯亚甲基(-CH=CH-CH=CH-CH2-)可能是可氧化位点(图1)。但如图10B所示,BKA对Cu2+氧化不敏感(而亚油酸LA可被氧化),且对15-LOX也具有抗性。200-800 nm波长范围内未检测到吸收峰(数据未显示),表明BKA比LA更耐受Cu2+介导的氧化。 ADP/ATP载体还能被米酵菌酸(BA)高效特异性抑制,这种由Pseudomonas cocovenenans细菌分泌的天然毒素是一种多不饱和长链脂肪酸衍生物(图2B)。BA以纳摩尔级亲和力(Kd)与线粒体基质侧载体位点结合,因此不同于ATRs,BA需穿过线粒体内膜才能抑制ADP/ATP转运。 研究表明ATRs与米酵菌酸/BA对ADP/ATP载体的结合互斥。此现象证明载体存在两种构象状态:能结合CATR的"CATR构象"和能结合BA的"BA构象"。这两种构象在线粒体膜中动态平衡,但CATR或BA会使平衡偏向形成稳定的CATR-载体或BA-载体复合物。这些复合物可通过化学/免疫化学反应性及蛋白酶敏感性区分。无抑制剂时,仅ADP/ATP能触发两种构象快速转换,表明该转变参与转运过程。此特性为在分子水平研究转运机制提供了独特优势。[2] |
| 体内研究 (In Vivo) |
Tempe bongkrek是印度尼西亚爪哇当地生产的廉价蛋白质来源。它是通过将椰奶或椰油生产的椰子肉副产品压成蛋糕,然后接种寡孢霉进行发酵制成的。最终产品被切成片或切成块用于煎炸或煮汤。如果发酵不完全,B. cocovenenans和Bongkrekic acid/BA可以增殖。1895年首次报道了与食用坦佩邦克有关的BA中毒死亡。自1975年以来,食用受污染的tempe bongkrek已导致近3000例BA中毒,其中至少150例死亡。在印度尼西亚,报告的BA中毒患者死亡率平均为60%。在1988年爆发后,禁止生产tempe bongkrek,但生产和偶尔爆发继续发生。
在中国东北,用于制作面包、面条和饺子的发酵玉米制品似乎是Bongkrekic acid/BA中毒的主要来源。在中国南方,吊姜糕与BA中毒事件有关。此外,在中国和其他亚洲国家消费的银耳蘑菇中,有一半可能被土壤中的cocovenenans污染。BA暴发通常发生在印度尼西亚和中国温暖的夏季。 2015年,亚洲以外首次报道了Bongkrekic acid/BA毒性的暴发。2015年在莫桑比克西北部爆发的疫情导致75人死亡,许多饮用pombe的人患病,pombe是一种自制的发酵玉米粉饮料(表1)。 在早期研究Bongkrekic acid/BA的细胞病理生理学中,Welling等人发现羊心脏组织中葡萄糖含量和细胞摄氧量呈剂量依赖性降低,并伴有乳酸积累和酸中毒。这些发现使他们假设BA抑制线粒体酶。后来的研究表明,BA是ANT的特异性配体,通过将ANT冻结在其“m”(基质取向)构象中来抑制转位酶。每1 mg线粒体蛋白中仅1 μmol BA就足以完全阻断ADP的磷酸化。在6 mmol ATP条件下,每1 mg线粒体蛋白约需要10 μmol BA才能完全阻断ATP的水解。其他也能抑制ANT的天然毒素包括白术苷、离白术苷、伪羧基白术苷、表白术苷、羧基白术苷、芳基叠氮白术苷、n-乙基马来酰亚胺、木耳酸和异戊二酸。 诊断测试: 检测B. cocovenenans和Bongkrekic acid/BA困难且不可靠。已从受污染的食物和呕吐物中分离出cocovenenans。可以使用Biologic GN2系统等商业检测试剂盒进行识别。cocovenenans最常用的鉴定方法是16S rDNA测序,但有时会错误地鉴定出cocovenenans的其他伯克氏菌病原体。B. cocovenenans可以通过毛细管电泳-单链构象多态性(CE-SSCP),微阵列分析或基于探针的细胞捕捞来鉴定。最可靠的方法可能是多重PCR协议。从泰国一名男子的淋巴腺样体和肺组织中分离到cocovenenans,并通过16s rDNA测序进行鉴定。我们未见其他从生物培养基中分离检测出cocovenenans的报道。 我们找不到任何已发表的检测生物培养基Bongkrekic acid/BA的报道,但环境样品中BA的存在和定量可以使用液体薄层色谱法、色谱-质谱法和高压液相色谱法[1]进行检测。 |
| 细胞实验 |
甲醛形成分析(MTS)。[3]
在MTS实验中,细胞以5×103细胞/孔的密度接种在96孔板上,并在4小时后引入FAs和Bongkrekic acid/BKA(单个浓度如图所示)。48小时孵育后,根据制造商的说明,使用CellTiter 96®水溶液细胞增殖试验分析细胞活力。试验化学品在适当的有机溶剂中制备,包括二甲基亚砜(DMSO)或乙醇。对照培养皿中添加了等量的溶剂,在使用的最终浓度下,载体对甲醛的形成没有可测量的影响。 活/死细胞分析。[3] 将MCF-7和LTED细胞以5×103细胞密度接种于200 μl细胞培养基的96孔板上,接种4 h后分别加入Bongkrekic acid/BKA(0.01、1和25 μM)。48小时孵育后,根据制造商的说明,使用活/死细胞染色试剂盒II分析活细胞和坏死细胞。采用glmax - multi检测系统检测钙黄素- am和乙啶同二聚体III (EthD-III)的荧光。在ltd细胞形态学检查中,使用徕卡dil倒置显微镜获得图像,使用Pixera®Penguin 600CL冷却CCD数码相机拍摄。数据处理采用Pixera Viewfinder 3.0软件。将乳腺癌细胞镀于6孔板上。在每口井中识别出三个细胞密度近似相等的区域,并捕获每个区域的图像。 转染和荧光素酶报告试验(双荧光素酶试验)。[3] 转染前一天,将MCF-7细胞(5×104 cells/well)接种于含有MEMα培养基的24孔板上。根据制造商的说明,使用Lipofectamine®LTX和PLUS™试剂转染每个表达质粒。人PPARγ表达质粒与人类视黄醇X受体α (RXRα)质粒结合的最大转录效率分别为100 ng和100 ng。将含有大鼠酰基辅酶a氧化酶PPRE的300 ng PPRE- luc质粒与20 ng Renilla荧光素酶报告质粒(pRL-CMV)的DNA混合物在24孔板上共转染。所有质粒浓度均与pcDNA3.1载体相等。人PPARγ、RXRα和PPRE报告基因表达质粒由Curtis J. Omiecinski博士赠送。转染24 h后,用磷酸盐缓冲盐水洗涤细胞,将细胞转化为不含酚红的MEMα,并添加5%血清,然后用Bongkrekic acid/BKA及其衍生物(BKA-1 ', BKA-2, BKA-3和BKA-4)处理24 h。用100 μl的被动裂解缓冲液制备细胞提取物,20 μl用于glmax - multi检测系统检测萤火虫荧光素酶和Renilla荧光素酶。每个样品中萤火虫荧光素酶活性(从报告质粒表达)与Renilla荧光素酶活性(从pRL-CMV表达)的比值作为标准化荧光素酶活性的测量。 共轭二烯形成的测定。[3] 实验是根据先前描述的程序进行的。Bongkrekic acid/BKA或LA在铜(Cu2+, CuSO4)存在下,在分光光度管(1.0 cm光程)中,在100mm硼酸缓冲液中,pH为9.0,室温下孵育不同时间。加入铜后,定期记录234 nm处的吸光度。结果表示为反应混合物在时间为零时吸光度的增加。 |
| 药代性质 (ADME/PK) |
Exposure [1]
Bongkrekic acid production depends on two distinct and sequential environmental conditions: those that support bacterial growth and proliferation, followed by those that favor Bongkrekic acid/BA production (Table 2). Bongkrekic acid is produced in warm environments (22–30 °C) with a neutral pH, the same conditions under which tempe is made. Production is also dependent on the presence of fatty acids, particularly those found in coconut and corn. Bacterial growth media containing oleic acid produced the highest concentrations of BA. When B. cocovenenans is cultured on coconut medium under ideal conditions, toxin production can reach 2–4 mg/g by the second day of culture. Lauric, myristic, and palmitic acids make up 71.5–74.5 % (by weight) of the fatty acids in coconut oil, and oleic acid can be found in varying concentrations in corn. Interestingly, R. oligosporum has a suppressing effect on BA production and can reduce BA concentration when allowed to form adequate numbers of fungal colonies. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicokinetics [1]
There is scarce information on the toxicokinetics and lethal dose of Bongkrekic acid/BA in humans. One source suggests that 1–1.5 mg can be fatal in humans and another suggests an oral LD50 of 3.16 mg/kg. Studies on mice suggest an oral LD50 of 0.68–6.84 mg/kg and an intravenous LD50 of 1.41 mg/kg. Another study in rats showed that a 2 mg/100 g oral dose caused death within 2–5 h. In the same study, rats survived an initial 1 mg/100 g, but a repeat dose after 48 h caused death. The absorption profile and volume of distribution for Bongkrekic acid/BA is unknown, although BA likely has a large volume of distribution because it is a highly unsaturated fat and is highly lipid soluble. We do not know how BA is metabolized. Early studies reported Flavotoxin A (a toxin also thought to be found in B. cocovenenans) and BA to be the same organic chemical compound according to nuclear magnetic resonance spectra, ultraviolet spectra, molar extinction coefficients, and mass spectra, but more recent studies theorize that flavotoxin A is possibly a metabolite of BA. The route of elimination of BA is unknown. Toxicity Summary IDENTIFICATION AND USE: Bongkrekic acid (BA) is a white amorphous solid. BA is known to be produced by the bacterium Burkholderia gladioli pv. cocovenenans. It is used as a tool in biochemical research. HUMAN STUDIES: In a rural town in Mozambique, >230 persons became sick and 75 died of an illness linked to drinking pombe, a traditional alcoholic beverage. Toxic levels of BA were detected in the suspect pombe but not the control pombe. Burkholderia gladioli pathovar cocovenenans, the bacteria that produces BA, was detected in the flour used to make the pombe. BA is an inhibitor of adenine nucleotide translocase (ANT). Since inhibition of ANT is connected to the inhibition of cytochrome c release from mitochondria, which then results in the suppression of apoptosis, it has been used as a tool for the mechanistic investigation of apoptosis. BA has been implicated in outbreaks of food-borne illness involving coconut- and corn-based products in Indonesia and China. ANIMAL STUDIES: BA, a potent inhibitor of the mitochondrial ATP/ADP translocase, inhibits glucose-induced electrical activity in the pancreatic beta-cell through the stimulation of ATP-sensitive potassium channel (K-ATP-channel) activity. mouse LD50 intravenous 1410 ug/kg Tetrahedron., 26(5993), 1970 mouse LDLo oral 6840 ug/kg BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: REGIDITY; LUNGS, THORAX, OR RESPIRATION: DYSPNEA Applied and Environmental Microbiology., 48(690), 1984 [PMID:6391376] Interactions The in vitro effect of bongkrekic acid on stem bromelain, papain and ficin was studied. The hydrolysis of casein by these enzymes was inhibited by bongkrekic acid, but the inhibition was always incomplete even with a large excess of the effector. Using a fully activated specimen of stem bromelain, purified on an organomercurial agarose affinity column, the inhibition by bongkrekic acid was not stoichiometric. The SH group of cysteine remained intact after incubation with an excess of bongkrekic acid at 24 degrees C for 20 min. However, partial inhibition of stem bromelain by bongkrekic acid was reversed by incubation at 37 degrees C for 5 min with 5 mM cysteine or 2-mercaptoethanol. Ethylene glycol and glycerol had no such restorative effect. These results indicate that molecules of bongkrekic acid are non-covalently bound to a thiol protease, only partially and reversibly shielding its essential SH group. Murachi T et al; Toxicon 20 (6): 1011-7 (1982) Antidote and Emergency Treatment /SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/ /SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/ /SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W TKO /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's (LR) if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam (Valium) or lorazepam (Ativan) ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/ Human Toxicity Excerpts /CASE REPORTS/ In January 2015, 75 people died and 177 were hospitalized in the Mozambique village of Chitima after attending a funeral. The deaths were linked to the consumption of a traditional African beverage called pombe. Samples of the suspect pombe were subjected to myriad analyses and compared to a control sample. Ultimately, non-targeted liquid chromatography-mass spectrometry screening revealed the presence of the potent toxin bongkrekic acid, and its structural isomer, isobongkrekic acid. Quantitative analysis found potentially fatal levels of these toxins in the suspect pombe samples. Bongkrekic acid is known to be produced by the bacterium Burkholderia gladioli pv. cocovenenans. This bacterium could not be isolated from the suspect pombe, but bacteria identified as B. gladioli were isolated from corn flour, a starting ingredient in the production of pombe, obtained from the brewer's home. When the bacteria were co-plated with the fungus Rhizopus oryzae, which was also isolated from the corn flour, synergistic production of bongkrekic acid was observed. The results suggest a mechanism for bongkrekic acid intoxication, a phenomenon previously thought to be restricted to specific regions of Indonesia and China. PMID:27823840 /CASE REPORTS/ BACKGROUND: On 9 January 2015, in a rural town in Mozambique, >230 persons became sick and 75 died of an illness linked to drinking pombe, a traditional alcoholic beverage. METHODS: An investigation was conducted to identify case patients and determine the cause of the outbreak. A case patient was defined as any resident of Chitima who developed any new or unexplained neurologic, gastrointestinal, or cardiovascular symptom from 9 January at 6:00 am through 12 January at 11:59 pm. We conducted medical record reviews, healthcare worker and community surveys, anthropologic and toxicologic investigations of local medicinal plants and commercial pesticides, and laboratory testing of the suspect and control pombe. RESULTS: We identified 234 case patients; 75 (32%) died and 159 recovered. Overall, 61% of case patients were female (n = 142), and ages ranged from 1 to 87 years (median, 30 years). Signs and symptoms included abdominal pain, diarrhea, vomiting, and generalized malaise. Death was preceded by psychomotor agitation and abnormal posturing. The median interval from pombe consumption to symptom onset was 16 hours. Toxic levels of bongkrekic acid (BA) were detected in the suspect pombe but not the control pombe. Burkholderia gladioli pathovar cocovenenans, the bacteria that produces BA, was detected in the flour used to make the pombe. CONCLUSIONS: We report for the first time an outbreak of a highly lethal illness linked to BA, a deadly food-borne toxin in Africa. Given that no previous outbreaks have been recognized outside Asia, our investigation suggests that BA might be an unrecognized cause of toxic outbreaks globally. PMID:29155976 /ALTERNATIVE and IN VITRO TESTS/ BACKGROUND/AIM: An in vitro cell model of long-term estrogen-deprived MCF-7 (LTED) cells has been utilized to analyze the re-growth mechanisms of breast cancers treated with blockers for estrogen receptor a (ERa) signaling. Bongkrekic acid (BKA) is a natural toxin isolated from coconut tempeh contaminated with the bacterium Burkholderia cocovenans. MATERIALS AND METHODS: LTED cells, MCF-7 cells and MDA-MB-231 cells were employed in the study. After treatment with BKA (chemically synthesized; purity: >98%), several biochemical analyses were carried out. RESULTS: LTED cells were categorized into an oxidative phenotype. When LTED cells were treated with BKA, lactate dehydrogenase A (LDH-A)/pyruvate dehydrogenase kinase 4 (PDK4) were down-regulated, thereby prompting the aggressive use of glucose via mitochondrial oxidative phosphorylation and induction of cell death responses. These effects of BKA were not observed in the other breast cancer cells analyzed. CONCLUSION: We suggest the potential of BKA as an experimental tool for the analysis of cancer biology in LTED cells. PMID:27798877 /ALTERNATIVE and IN VITRO TESTS/ Bongkrekic acid (BKA) is an inhibitor of adenine nucleotide translocase (ANT). Since inhibition of ANT is connected to the inhibition of cytochrome c release from mitochondria, which then results in the suppression of apoptosis, it has been used as a tool for the mechanistic investigation of apoptosis. BKA consists of a long carbon chain with two asymmetric centers, a nonconjugated olefin, two conjugated dienes, three methyl groups, a methoxyl group, and three carboxylic acids. This complicated chemical structure has caused difficulties in synthesis, supply, and biochemical mechanistic investigations. In this study, we designed and synthesized more simple tricarboxylic acids that were inspired by the molecular structure of BKA. Their cytotoxicity and apoptosis-preventing activity in HeLa cells and the effect on the mitochondrial inner membrane potential in HL-60 cells were then evaluated. All tested tricarboxylic acid derivatives including BKA showed little toxicity against HeLa cells. BKA and two of the synthesized derivatives significantly suppressed staurosporine (STS)-induced reductions in cell viability. Furthermore, STS-induced /mitochondrial potential/ collapse was significantly restored by pretreatment with BKA and a tricarboxylic acid derivative. Other derivatives, in which one of three carboxylic acids was esterified, exhibited potent toxicity, especially a derivative bearing a carbon chain of the same length as that of BKA. In conclusion, we have developed a new ... compound as an apoptosis inhibitor bearing three carboxylic acids connected with the proper length of a long carbon chain. PMID:22998163 Non-Human Toxicity Excerpts /ALTERNATIVE and IN VITRO TESTS/ Bongkrekic acid causes fatal food poisoning which is associated with hyperglycemia. Here we demonstrate that bongkrekic acid, a potent inhibitor of the mitochondrial ATP/ADP translocase, inhibits glucose-induced electrical activity in the pancreatic beta-cell through the stimulation of ATP-sensitive potassium channel (K-ATP-channel) activity. By comparison of its effects with those of oligomycin, we suggest that bongkrekic acid acts by the inhibition of glucose metabolism and may induce hyperglycemia by impairing beta-cell function. PMID:2037079 /ALTERNATIVE and IN VITRO TESTS/ The aim of this work was to characterize the effect of bongkrekic acid (BKA), atractyloside (ATR) and carboxyatractyloside (CAT) on single channel properties of chloride channels from mitochondria. Mitochondrial membranes isolated from a rat heart muscle were incorporated into a bilayer lipid membrane (BLM) and single chloride channel currents were measured in 250/50 mM KCl cis/trans solutions. BKA (1-100 uM), ATR and CAT (5-100 uM) inhibited the chloride channels in dose-dependent manner. The inhibitory effect of the BKA, ATR and CAT was pronounced from the trans side of a BLM and it increased with time and at negative voltages (trans-cis). These compounds did not influence the single channel amplitude, but decreased open dwell time of channels. The inhibitory effect of BKA, ATR and CAT on the mitochondrial chloride channel may help to explain some of their cellular and/or subcellular effects. PMID:17123460 /OTHER TOXICITY INFORMATION/ Two distinct conformations of the mitochondrial ADP/ATP carrier involved in the adenine nucleotide transport are called BA and CATR conformations, as they were distinguished by binding of specific inhibitors bongkrekic acid (BA) and carboxyatractyloside (CATR), respectively. To find out which amino acids are implicated in the transition between these two conformations, which occurs during transport, mutants of the Saccharomyces cerevisiae ADP/ATP carrier Anc2p responsible for resistance of yeast cells to BA were identified and characterized after in vivo chemical or UV mutagenesis. Only four different mutations could be identified in spite of a large number of mutants analyzed. They are located in the Anc2p transmembrane segments I (G30S), II (Y97C), III (L142S), and VI (G298S), and are independently enabling growth of cells in the presence of BA. The variant and wild-type Anc2p were produced practically to the same level in mitochondria, as evidenced by immunochemical analysis and by atractyloside binding experiments. ADP/ATP exchange mediated by Anc2p variants in isolated mitochondria was more efficient than that of the wild-type Anc2p in the presence of BA, confirming that BA resistance of the mutant cells was linked to the functional properties of the modified ADP/ATP carrier. These results suggest that resistance to BA is caused by alternate conformation of Anc2p due to appearance of Ser or Cys at specific positions. Different interactions of these residues with other amino acids and/or BA could prevent formation of stable inactive Anc2p BA complex. PMID:13678275 /OTHER TOXICITY INFORMATION/ The interactions between aflatoxin-producing fungi and bacteria have opened up a new avenue for identifying biological agents suitable for controlling aflatoxin contamination. In this study, we analyzed the interactions between A. flavus and the bacterium Burkholderia gladioli M3 that coexist in rice that is naturally contaminated with A. flavus. Our results showed that a cell-free culture filtrate (CCF) and the metabolite bongkrekic acid of the M3 strain potently suppressed the mycelial growth and spore production, and then affected the production of aflatoxin of A. flavus. Bongkrekic acid secreted by the M3 strain exhibited higher antifungal activity than did analogues. The CCF of the M3 strain and its metabolite bongkrekic acid can inhibit the growth of A. flavus, but the metabolites of A. flavus, aflatoxins, exerted no inhibitory effect on the growth of the M3 strain. Furthermore, we determined that the M3 cells could use the dead mycelia of A. flavus as energy sources for reproduction, while A. flavus could not grow in a solution containing dead M3 cells. In summary, these results indicated that B. gladioli has a competitive advantage in survival when it coexists with its fungal partner A. flavus. PMID:26058536 Non-Human Toxicity Values LD50 Mice iv 1.4 mg/kg PMID:10435074 LD50 Mice oral 3.16 mg/kg /Purified Flavotoxin A/ |
| 参考文献 |
|
| 其他信息 |
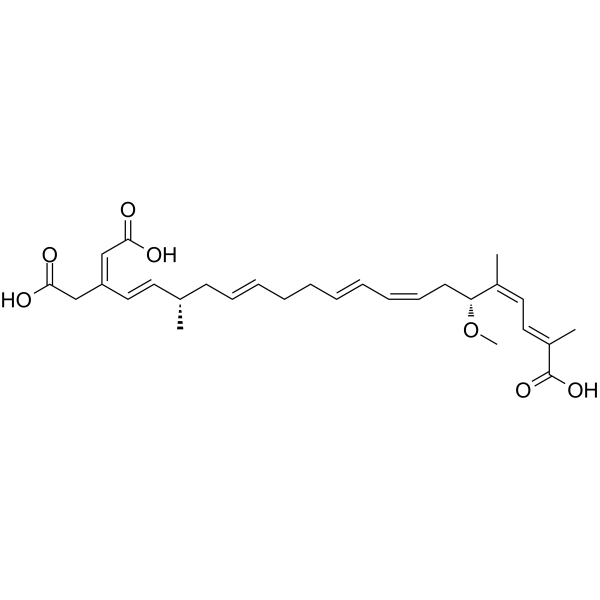
Bongkrekic acid is a tricarboxylic acid that is docosa-2,4,8,10,14,18,20-heptaenedioic acid substituted at positions 2 ,5 and 17 by methyl groups, at positions 6 by a methoxy group and at position 20 by a carboxymethyl group. It is produced by the bacterium Burkholderia gladioli and implicated in outbreaks of food-borne illness involving coconut and corn-based products in Indonesia and China. It has a role as an apoptosis inhibitor, an EC 2.5.1.18 (glutathione transferase) inhibitor, a toxin, an ATP/ADP translocase inhibitor and a bacterial metabolite. It is a tricarboxylic acid, an ether and an olefinic compound. It is a conjugate acid of a bongkrekate(3-).
Bongkrekic acid has been reported in Burkholderia gladioli with data available. An antibiotic produced by Pseudomonas cocovenenans. It is an inhibitor of MITOCHONDRIAL ADP, ATP TRANSLOCASES. Specifically, it blocks adenine nucleotide efflux from mitochondria by enhancing membrane binding. Mechanism of Action Bongkrekic acid (BKA) is an inhibitor of adenine nucleotide translocase (ANT). Since inhibition of ANT is connected to the inhibition of cytochrome c release from mitochondria, which then results in the suppression of apoptosis, it has been used as a tool for the mechanistic investigation of apoptosis. BKA consists of a long carbon chain with two asymmetric centers, a nonconjugated olefin, two conjugated dienes, three methyl groups, a methoxyl group, and three carboxylic acids. This complicated chemical structure has caused difficulties in synthesis, supply, and biochemical mechanistic investigations. Bongkrekic acid (BA) has a unique mechanism of toxicity among the mitochondrial toxins: it inhibits adenine nucleotide translocase (ANT) rather than the electron transport chain. Bongkrekic acid is produced by the bacterium Burkholderia gladioli pathovar cocovenenans (B. cocovenenans) which has been implicated in outbreaks of food-borne illness involving coconut- and corn-based products in Indonesia and China. Introduction: Bongkrekic acid (BA) has a unique mechanism of toxicity among the mitochondrial toxins: it inhibits adenine nucleotide translocase (ANT) rather than the electron transport chain. Bongkrekic acid is produced by the bacterium Burkholderia gladioli pathovar cocovenenans (B. cocovenenans) which has been implicated in outbreaks of food-borne illness involving coconut- and corn-based products in Indonesia and China. Our objective was to summarize what is known about the epidemiology, exposure sources, toxicokinetics, pathophysiology, clinical presentation, and diagnosis and treatment of human BA poisoning. Methods: We searched MEDLINE (1946 to present), EMBASE (1947 to present), SCOPUS, The Indonesia Publication Index ( http://id.portalgaruda.org/ ), ToxNet, book chapters, Google searches, Pro-MED alerts, and references from previously published journal articles. We identified a total of 109 references which were reviewed. Of those, 29 (26 %) had relevant information and were included. Bongkrekic acid is a heat-stable, highly unsaturated tricarboxylic fatty acid with a molecular weight of 486 kDa. Outbreaks have been reported from Indonesia, China, and more recently in Mozambique. Very little is known about the toxicokinetics of BA. Bongkrekic acid produces its toxic effects by inhibiting mitochondrial (ANT). ANT can also alter cellular apoptosis. Signs and symptoms in humans are similar to the clinical findings from other mitochondrial poisons, but they vary in severity and time course. Management of patients is symptomatic and supportive. Conclusions: Bongkrekic acid is a mitochondrial ANT toxin and is reported primarily in outbreaks of food-borne poisoning involving coconut and corn. It should be considered in outbreaks of food-borne illness when signs and symptoms manifest involving the liver, brain, and kidneys and when coconut- or corn-based foods are implicated. [1] The ADP/ATP carrier plays a key role in cell economy. Because of its unique properties, it has provided, in biochemical and genetic studies, advanced insights into the molecular basis of metabolite transport across biomembranes. A major progress in the knowledge of this carrier results from the recent determination at high resolution of the structure of the CATR-carrier complex. Solving the structure of the carrier in other conformational states would provide essential information to elucidate the molecular mechanism of adenine nucleotide exchange across the inner mitochondrial membrane and highlight the consequences of mutations involved in related genetic diseases. In providing the cell with ATP generated by oxidative phosphorylation, the mitochondrial ADP/ATP carrier plays a central role in aerobic eukaryotic cells. Combining biochemical, genetic, and structural approaches contributes to understanding the molecular mechanism of this essential transport system, the dysfunction of which is implicated in neuromuscular diseases. [2] In the present study, we did not obtain direct evidence for the interaction between the Bongkrekic acid/BKA-induced down-regulation of LDH-A/PDK4 (Topo IIα/Ki-67) and LTED cell death; however, this is the first study to identify BKA as a highly selective modulator for the metabolic pathway in LTED cells (Figure 5B). BKA has potential as a therapeutic modality for the recurrence of breast cancers that have already been treated with blockers of 17β-estradiol/ERα signaling; however, further investigations are needed on the mechanism(s) responsible for BKA-mediated cell death in LTED cells.[3] |
| 分子式 |
C28H38O7
|
|---|---|
| 分子量 |
486.59712
|
| 精确质量 |
486.262
|
| CAS号 |
11076-19-0
|
| PubChem CID |
6433556
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
1.114g/cm3
|
| 沸点 |
715.1ºC at 760mmHg
|
| 熔点 |
50-60°
|
| 闪点 |
231ºC
|
| 折射率 |
1.545
|
| LogP |
5.885
|
| tPSA |
121.13
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
17
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
898
|
| 定义原子立体中心数目 |
2
|
| SMILES |
OC(C/C(/C=C/[C@H](C/C=C/CC/C=C/C=C/C[C@H](/C(=C/C=C(/C(=O)O)\C)/C)OC)C)=C\C(=O)O)=O
|
| InChi Key |
SHCXABJSXUACKU-WUTQZGRKSA-N
|
| InChi Code |
InChI=1S/C28H38O7/c1-21(15-18-24(19-26(29)30)20-27(31)32)13-11-9-7-5-6-8-10-12-14-25(35-4)22(2)16-17-23(3)28(33)34/h6,8-12,15-19,21,25H,5,7,13-14,20H2,1-4H3,(H,29,30)(H,31,32)(H,33,34)/b8-6+,11-9+,12-10-,18-15+,22-16-,23-17+,24-19+/t21-,25+/m0/s1
|
| 化学名 |
(2E,4Z,6R,8Z,10E,14E,17S,18E,20Z)-20-(carboxymethyl)-6-methoxy-2,5,17-trimethyldocosa-2,4,8,10,14,18,20-heptaenedioic acid
|
| 别名 |
BONGKREKIC ACID; 11076-19-0; Flavotoxin A; Bongkrek acid; L7V4I673D2; (2E,4Z,6R,8Z,10E,14E,17S,18E,20Z)-20-(carboxymethyl)-6-methoxy-2,5,17-trimethyldocosa-2,4,8,10,14,18,20-heptaenedioic acid; (-)-BONGKREKIC ACID; BONGKREKIC ACID [MI];
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0551 mL | 10.2754 mL | 20.5508 mL | |
| 5 mM | 0.4110 mL | 2.0551 mL | 4.1102 mL | |
| 10 mM | 0.2055 mL | 1.0275 mL | 2.0551 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。