| 规格 | 价格 | ||
|---|---|---|---|
| 5mg | |||
| Other Sizes |
| 靶点 |
Plasmepsin IX/X (PMIX/X); Plasmodium
|
|---|---|
| 体外研究 (In Vitro) |
WM382 抑制恶性疟原虫和间日疟原虫,对恶性疟原虫的 IC50 值为 0.6 nM,并对 HepG2 细胞表现出相当大的细胞毒性 (IC50=24.8 μM)[2][3]。 WM382 的 Ki 值分别为 13.4 μM 和 0.035 nM,选择性结合 PMV 和 PMX[3]。在伯氏疟原虫感染的 HepG2 体外培养物中,WM382(1 nM 和 100 nM)提醒注射后达到血液感染的时间[3]。
|
| 体内研究 (In Vivo) |
在伯氏疟原虫和恶性疟原虫寄生虫小鼠模型中,WM382(20 mg/kg,每日两次或 1-30 mg/kg,每日一次;口服;持续 4 天)可有效消除感染。 WM382 可以有效治疗人源化小鼠的无性恶性疟原虫感染,同时还能阻止蚊子传播[3]。
|
| 酶活实验 |
恶性疟原虫白酶加工抑制试验[2]
蛋 加工抑制试验基本上按照之前的描述进行(Favuzza等人,2020)。将蛋白酶抑制剂添加到同步的后期滋养体/早期分裂体培养物中,然后通过LD或LS磁柱去除未感染的红细胞。WM4和WM382分别以40 nM和2.5 nM的终浓度加入。不含蛋白酶抑制剂的对照盘。用含有相同浓度抑制剂的完整RPMI 1640培养基洗脱柱上的寄生虫。将洗脱的寄生虫调整为5 × 106个/mL,每孔添加150 mL, 96孔平底培养皿。寄生虫培养16小时,并涂片代表性孔进行吉氏染色,以确保破裂正常发生(对照孔)或破裂被阻断(WM4, WM382>条件)。寄生虫以10000 g/10 min离心,收集分殖子和上清。将两个部分的蛋白质加入还原样品缓冲液中,用4-12%或3-8%的丙烯酰胺凝胶分离,用于后续的免疫印迹。 表面等离子体共振[2] PvPMX或PfPMX蛋白酶通过胺偶联固定在CM5传感器芯片上,在10 mM激活的pH 5.5中,以10 mL min - 1的流速注射420 s,通常固定约4000 RU的蛋白质。所有实验均在HBS-EP缓冲液(10 mM Hepes pH 7.4, 150 mM NaCl, 3 mM EDTA和0.05%吐温20)中进行,温度为18°C,流速为30 mL min - 1。将化合物WM4和WM382在HBS-EP +缓冲液中稀释至0.031 nM - 16 nM范围内的适当浓度。接触时间为90 s,分离时间为1500 s,然后用50 mM甘氨酸pH 9.5进行30 s再生。使用新固定的传感器表面独立进行了三次实验。PvPMX数据采集于BIAcore S200仪器,数据分析软件版本为BIAcore S200 1.1; PfPMX数据采集于BIAcore 8K+仪器,数据分析软件版本为BIAcore Insight 3.0.12.15655。所有传感器图均采用活化的乙醇胺阻断传感器表面和HBS-EP +缓冲空白进行双重参考。使用1:1的结合模型拟合WM4的打开和关闭率来确定亲和力,由于关闭率太慢,无法报告WM382的亲和力。 CETSA热稳定性测定[3] Lysate CETSA实验基本上按照描述进行(Dziekan et al., 2019)。CETSA研究样品采用高度同步分裂期恶性疟原虫3D7-PMIX_HA和3D7-PMX_HA寄生虫制备。后期寄生虫(入侵后40-44小时)通过Percoll密度梯度纯化,并与1 μM化合物1 (C1)抑制剂(内部合成)孵育(Hale等,2017)。孵育2-4 h后,成熟分裂体用PBS洗涤1次,感染的红细胞在20体积0.15% (w/v)皂苷的PBS中裂解,冰孵育20分钟。寄生虫在冰冷的PBS中洗涤3次,最终的球在10体积裂解缓冲液(0.4% NP-40 (Roche) / PBS)中重悬,通过3次冷冻(干冰/乙醇浴)-解冻循环裂解。裂解物在16000 g离心30分钟后清除,含有可溶性寄生虫蛋白的上清液在−80℃保存至使用。将化合物601 (5 μM)、WM4 (2 μM)和WM382 (1 μM)加入到8 × 50 μg蛋白裂解液等分液中(蛋白质浓度通过BCA蛋白测定法测定),室温(RT)孵育3min,在Biorad T100热循环器中分别在65-40℃温度梯度下加热3min,然后在4℃孵育3min。加热后的裂解物在4℃下13000 g离心30分钟。根据制造商的说明,在预制的4%-12%梯度凝胶上用MES运行缓冲液溶解可溶性蛋白质。 |
| 细胞实验 |
敲除疟原虫的EC50测定[3]
培养环期恶性疟原虫3D7、3D7- pmix - ha和3D7- pmix - ha,同时增加GlcN (Sigma)浓度。37℃孵育72 h后,用0.06%皂苷溶解滋养体感染的红细胞,用2倍还原SDS-PAGE样品缓冲液溶解微球,进行抗ha免疫检测。如前面所述,在2.5 nM GlcN(分别是ha标记蛋白的正常表达和减少表达)缺失和存在的情况下,通过FACS和SYBR Green测定WM4和WM382对恶性疟原虫3D7、3D7- pmix - ha和3D7- pmx - ha寄生虫的抑制EC50。 药物杀伤时间[3] 用5%山梨醇(Sigma)同步3D7寄生虫培养,每隔46 h同步两次,然后当培养中混合了晚分裂体和早分裂体时再次同步。在3%环数和4%红细胞压积的条件下,分别建立3份10ml的培养液,分别含有80nM WM4、40nM WM5、5nM WM382或DMSO (Sigma)载体对照。每个培养物每8 h定量一次,共48 h,采集50 μL样品,流式细胞术计数(如前所述)。用吉姆萨染色血膜显微镜检查各时间点寄生虫的发育阶段。在32 h时间点更换培养基和化合物。 入侵检测[3] 山梨醇处理与环期添加WM4 (40 nM)、WM382 (2.5 nM)或DMSO对照同步培养3D7寄生虫。后期寄生体(入侵后40 h)通过磁分离富集,发育为完全节段的分裂体寄生体。为了防止分裂体破裂和分裂子释放,对照寄生虫用1 μM化合物1 (C1)孵育。孵育5-6小时后,将寄生液泡膜封闭的分生子(PEMS)制成颗粒,在小体积完整培养基(含WM4, WM382或C1)中重悬,并通过1.2 μm注射器过滤器(Acrodisc;32毫米;棺罩)。将含有纯化的分裂子的滤液立即加入新鲜红细胞(70%-80%的红细胞比容)中,在摇床(1100转/分)中37℃孵育20分钟,使宿主细胞浸润,然后稀释至2%的红细胞比容。37℃孵育24小时后,通过显微镜(giemsa染色薄涂片)和流式细胞术测量寄生虫率来评估侵袭情况 电阻时间的测定[3] 实验1将105、106、107、108和109株Dd2寄生虫分别暴露于10 nM的atovaquone或1.5 nM WM382环境中,监测90 d。实验2将106、107和108 Dd2寄生虫的三次培养分别暴露于5 nM的atovaquone、80 nM的WM5或1.5 nM WM382中,监测62天。每个实验都通过每周一次的血膜显微镜检查来监测。每周更换三次培养基和化合物。 |
| 动物实验 |
Animal/Disease Models: Mice infected with P. berghei[3]
Doses: 20 mg/kg Route of Administration: po (oral gavage); twice (two times) daily for 4 days; monitored for 30 days Experimental Results: Cured mice of P. berghei and prevents blood infection from the liver. Animal/Disease Models: Humanized nonobese diabetic-severe combined immunodeficiency (NOD-scid) IL2Rgnull mouse model (NSG)[3] Doses: 1, 3, 10, 30 mg/kg Route of Administration: po (oral gavage); one time/day for 4 days; monitored for 7 days Experimental Results: Cleared of parasitemia by day 6 at 30 mg/kg or day 7 at 3 and 10 ma/kg. Dose Ranging Test for P. berghei Infection in Mice [3] In the dose ranging studies, mice were treated orally with WM382 for 4 days by a b.i.d. dosing regimen at 30, 10, 3 or 1 mpk/day (Figure 4B), or by a q.d. dosing regimen at 60, 30 or 10 mpk/day (Figure 4C), with the first dose given 2 h after infection. Control mice were treated orally with chloroquine for 4 days under q.d. dosing regimen at 10 mpk/day. From day 2 to 30 post infection, parasitemia was measured daily by flow cytometry and microscopy, as described above. Survival of animals to day 30 post infection, with no detectable parasites in the peripheral blood, were considered to be cured (i.e., 100% efficacy). P. falciparum Humanized NOD-scid IL2R_null Mouse Model [3] Compounds were tested in the murine P. falciparum SCID model (Angulo-Barturen et al., 2008). Briefly, WM382 formulated in 20% DMSO, 60% propylene glycol and 20% water, was administered to a cohort of age-matched female immunodeficient NOD-scid IL- 2Rγnull mice previously engrafted with human erythrocytes. The mice were intravenously infected with 2 × 107 P. falciparum Pf3D7-infected erythrocytes (day 0) (Angulo-Barturen et al., 2008). On day 3 after infection, mice were randomly allocated to treatments that were administered once a day for 4 consecutive days (n = 3 mice) by oral gavage at 10 mL/kg. Parasitemia was measured by microscopy. Chimerism was monitored by flow cytometry using anti-murine erythrocyte TER119 monoclonal antibody and SYTO-16 and then analyzed by flow cytometry in serial 2 μL blood samples taken every 24 h until assay completion. In Vivo Analysis of P. berghei Liver Parasite Growth and Transition to Blood Infection [3] Female BALB/c mice were infected with PbmCherryLuci sporozoites by either intravenous injection or infectious mosquito bite challenge. Mice (infected by either route) received either no treatment or were treated orally with WM382 (prepared as previously described) at doses and h post infection (hpi) as indicated. For i.v. injections, freshly isolated salivary gland sporozoites were filtered through glass wool to remove debris before 40,000 sporozoites were resuspended in 200 μL Schneider’s Insect Media immediately prior to injection. For infection by mosquito bite, the percent of mosquitoes that contained oocysts was used to place 5 infected mosquitoes in individual feeding cups (for example, if 83% of mosquitoes in the batch had oocysts then 6 mosquitoes were placed in each cup). Mice were anesthetised using ketamine/xylazine and placed on individual feeding cups to begin the infection. Mosquitoes were allowed to feed on the mice for 15 min and mice were rotated between feeding cups every min to promote probing and ensure that all mice were equally exposed to infectious mosquito bites. From day 3 – 30 post infection mice were monitored daily for the presence of parasites in Giemsa stained thin blood smears and considered to be protected from blood infection if they remained negative through this period. Peters’ 4-Day Suppressive Test for P. berghei Infection in Mice [3] For results shown in Figure 1 A male Swiss mice were infected intraperitoneally (IP) with 1 × 106 P. berghei ANKA-parasitised red blood cells withdrawn from a previously infected donor mouse. Test compounds were prepared in a vehicle consisting of 10% DMSO/90% Solutol (5% Solutol® HS-15 in 0.9% saline). Two h post infection, mice were treated on 4 consecutive days (q.d. regimen, once a day) with an IP dose of test compounds (WM4, WM5: 20 mpk) or chloroquine (10 mpk), or received an IP injection of vehicle as a control. Peripheral blood samples were taken 24 h after administration of the last dose, and parasitemia was measured by microscopic analysis of Giemsa-stained blood smears. Parasitemia values were averages for 6 mice per group and are expressed as percent parasitemia. For results shown in Figure 4A ‘donor’ female Swiss mice were infected intraperitoneally (IP) with blood stage P. berghei parasites constitutively expressing GFP (P. berghei ANKA GFPcon 259cl2 (Franke-Fayard et al., 2004). Three days later, groups of 4 ‘acceptor’ Swiss mice were infected intravenously (IV) with 1 × 107 parasitised erythrocytes from the ‘donor’ mice. Two h post infection, experimental mice (4 per cohort) were left untreated (control mice) or treated orally on 4 consecutive days with test drugs formulated in 20% DMSO/60% PG/20% water (v/v/v) or chloroquine dissolved in water. Mice were treated with WM382 for 4 days by a b.i.d. dosing regimen (twice a day) at 20 mpk/day, with the first dose given 2 h after infection. Peripheral blood samples were taken 12 h after the last treatment, and parasitemia measured by flow cytometry (proportion of GFP-positive cells in 100,000 recorded events using FACSCalibur, BD) and microscopic analysis of Giemsa-stained blood smears. Parasitemia values were averages for 4 mice per group and are expressed as percent parasitemia. |
| 药代性质 (ADME/PK) |
Oral bioavailability (F=8% in rat and 38% in mice)
|
| 毒性/毒理 (Toxicokinetics/TK) |
Plasma protein binding = 95%
|
| 参考文献 |
[1]. Manuel de LR, et al. The Invention of WM382, a Highly Potent PMIX/X Dual Inhibitor toward the Treatment of Malaria. ACS Med Chem Lett. 2022 Oct 12.
[2]. Hodder AN, et al. Basis for drug selectivity of plasmepsin IX and X inhibition in Plasmodium falciparum and vivax. Structure. 2022 Jul 7;30(7):947-961.e6. [3]. Favuzza P, et al. Dual Plasmepsin-Targeting Antimalarial Agents Disrupt Multiple Stages of the Malaria Parasite Life Cycle. Cell Host Microbe. 2020 Apr 8;27(4):642-658.e12. |
| 其他信息 |
Drug resistance to first-line antimalarials─including artemisinin─is increasing, resulting in a critical need for the discovery of new agents with novel mechanisms of action. In collaboration with the Walter and Eliza Hall Institute and with funding from the Wellcome Trust, a phenotypic screen of Merck’s aspartyl protease inhibitor library identified a series of plasmepsin X (PMX) hits that were more potent than chloroquine. Inspired by a PMX homology model, efforts to optimize the potency resulted in the discovery of leads that, in addition to potently inhibiting PMX, also inhibit another essential aspartic protease, plasmepsin IX (PMIX). Further potency and pharmacokinetic profile optimization efforts culminated in the discovery of WM382, a very potent dual PMIX/X inhibitor with robust in vivo efficacy at multiple stages of the malaria parasite life cycle and an excellent resistance profile.[1]
Plasmepsins IX (PMIX) and X (PMX) are essential aspartyl proteases for Plasmodium spp. egress, invasion, and development. WM4 and WM382 inhibit PMIX and PMX in Plasmodium falciparum and P. vivax. WM4 inhibits PMX, while WM382 is a dual inhibitor of PMIX and PMX. To understand their function, we identified protein substrates. Enzyme kinetic and structural analyses identified interactions responsible for drug specificity. PMIX and PMX have similar substrate specificity; however, there are distinct differences for peptide and protein substrates. Differences in WM4 and WM382 binding for PMIX and PMX map to variations in the S' region and engagement of the active site S3 pocket. Structures of PMX reveal interactions and mechanistic detail of drug binding important for development of clinical candidates against these targets.[2] Artemisin combination therapy (ACT) is the main treatment option for malaria, which is caused by the intracellular parasite Plasmodium. However, increased resistance to ACT highlights the importance of finding new drugs. Recently, the aspartic proteases Plasmepsin IX and X (PMIX and PMX) were identified as promising drug targets. In this study, we describe dual inhibitors of PMIX and PMX, including WM382, that block multiple stages of the Plasmodium life cycle. We demonstrate that PMX is a master modulator of merozoite invasion and direct maturation of proteins required for invasion, parasite development, and egress. Oral administration of WM382 cured mice of P. berghei and prevented blood infection from the liver. In addition, WM382 was efficacious against P. falciparum asexual infection in humanized mice and prevented transmission to mosquitoes. Selection of resistant P. falciparum in vitro was not achievable. Together, these show that dual PMIX and PMX inhibitors are promising candidates for malaria treatment and prevention.[3] |
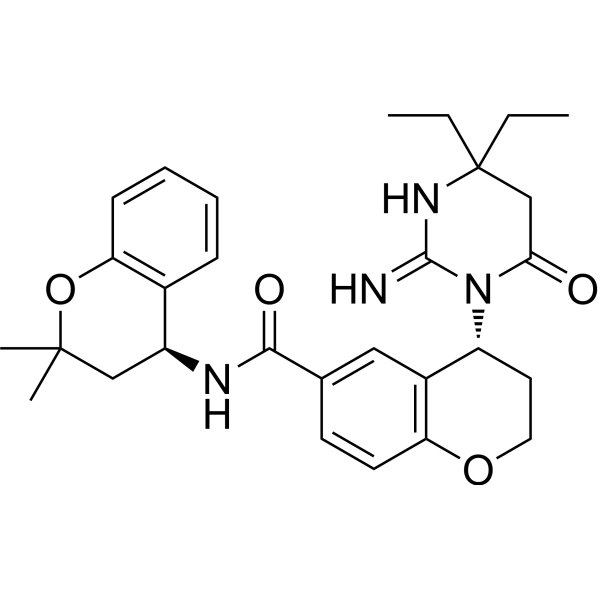
| 分子式 |
C29H36N4O4
|
|---|---|
| 分子量 |
504.620547294617
|
| 精确质量 |
504.273
|
| 元素分析 |
C, 69.02; H, 7.19; N, 11.10; O, 12.68
|
| CAS号 |
2606990-92-3
|
| PubChem CID |
154699453
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
3.4
|
| tPSA |
106
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
900
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CCC1(CC(=O)N(C(=N1)N)[C@@H]2CCOC3=C2C=C(C=C3)C(=O)N[C@H]4CC(OC5=CC=CC=C45)(C)C)CC
|
| InChi Key |
ZSZSSSHEMYULPX-FCHUYYIVSA-N
|
| InChi Code |
InChI=1S/C29H36N4O4/c1-5-29(6-2)17-25(34)33(27(30)32-29)22-13-14-36-23-12-11-18(15-20(22)23)26(35)31-21-16-28(3,4)37-24-10-8-7-9-19(21)24/h7-12,15,21-22H,5-6,13-14,16-17H2,1-4H3,(H2,30,32)(H,31,35)/t21-,22+/m0/s1
|
| 化学名 |
(4R)-4-(2-amino-4,4-diethyl-6-oxo-5H-pyrimidin-1-yl)-N-[(4S)-2,2-dimethyl-3,4-dihydrochromen-4-yl]-3,4-dihydro-2H-chromene-6-carboxamide
|
| 别名 |
WM-382; WM382; 2606990-92-3; CHEMBL5171401; (4R)-4-(2-amino-4,4-diethyl-6-oxo-5H-pyrimidin-1-yl)-N-[(4S)-2,2-dimethyl-3,4-dihydrochromen-4-yl]-3,4-dihydro-2H-chromene-6-carboxamide; (4R)-4-[(2E)-4,4-diethyl-2-imino-6-oxo-1,3-diazinan-1-yl]-N-[(4S)-2,2-dimethyl-3,4-dihydro-2H-1-benzopyran-4-yl]-3,4-dihydro-2H-1-benzopyran-6-carboxamide; I0L; SCHEMBL22997111; GTPL11162; WM 382
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 5 mg/mL (9.91 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9817 mL | 9.9084 mL | 19.8169 mL | |
| 5 mM | 0.3963 mL | 1.9817 mL | 3.9634 mL | |
| 10 mM | 0.1982 mL | 0.9908 mL | 1.9817 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。