| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
AdipoR1 (Kd = 1.8 μM); AdipoR2 (Kd = 3.1 μM)[1]
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
AdipoRon 盐酸盐是一种口服活性、选择性 AdipoR 激动剂,与 AdipoR1 和 AdipoR2 结合,Kds 分别为 1.8 和 3.1 μM。 AdipoRon (50 nM-50 μM) 通过 AdipoR1 刺激 AMPK 磷酸化 [1]。 AdipoRon (50 μM) 以剂量依赖性方式降低 L02 细胞中 TNF-α 和 TGF-β1 水平。 AdipoRon 对巨噬细胞具有显着且剂量依赖性的生长抑制作用[2]。 AdipoRon 治疗可增强再灌注后心脏功能的恢复并抑制 MI 后的细胞凋亡 [3]。 AdipoRon 通过与脂联素不同的机制引起血管舒张,并且不会显着降低 VSMC [Ca2+]i[4]。
|
||
| 体内研究 (In Vivo) |
AdipoRon(50 mg/kg,静脉注射)显着磷酸化野生型小鼠肝脏和骨骼肌中的 AMPK,但在双敲除 Adipor1−/− 或 Adipor2−/− 小鼠中则不然 [1]。 AdipoRon(0.02、0.1 和 0.5 mg/kg,ig)可防止 D-GalN 攻击引起的肝脏结构变形,并降低 D-GalN 在小鼠中诱导的肝毒性。在较大剂量(0.1 和 0.5 mg/kg)时,AdipoRon 的保肝作用尤其明显 [2]。在缺乏 APN 的小鼠中,AdipoRon(50 mg/kg,口服)可防止心肌细胞凋亡增加。在 AMPK-DN 小鼠中,AdipoRon 的抗凋亡作用减弱,但并未完全消除 [3]。
脂肪细胞分泌的脂联素与脂联素受体AdipoR1和AdipoR2结合,分别通过激活AMPK和PPAR-α途径发挥降糖作用。肥胖会导致血浆中的脂联素水平降低,从而导致胰岛素抵抗和2型糖尿病。因此,结合并激活AdipoR1和AdipoR2的口服活性小分子可以改善肥胖相关疾病,如2型糖尿病。在这里,我们报告了口服活性合成小分子AdipoR激动剂的鉴定。其中一种化合物,AdipoRon (AdipoRon),在体外结合AdipoR1和AdipoR2。AdipoRon在肌肉和肝脏中表现出与脂联素非常相似的作用,如激活AMPK和PPAR-α途径,并改善高脂饮食小鼠的胰岛素抵抗和葡萄糖耐受不良,而在AdipoR1和AdipoR2双敲除小鼠中完全消失。此外,AdipoRon改善了遗传肥胖的啮齿动物模型db/db小鼠的糖尿病,并延长了高脂肪饮食的db/db小鼠的寿命。因此,口服活性AdipoRon等AdipoRon激动剂是治疗肥胖相关疾病(如2型糖尿病)的一种有前景的治疗方法。[1] 脂联素是一种抗糖尿病和抗动脉粥样硬化的脂肪因子,在能量平衡中起着独特的作用。脂联素作为胰岛素增敏激素,通过特异性受体AdipoR1和AdipoR2,通过活化amp活化蛋白激酶(AMPK)和过氧化物酶体增殖物活化受体(PPAR)α途径发挥多种生物学作用。AdipoRon是一种口服活性的合成小分子AdipoRon激动剂,其体外和体内的作用与脂联素非常相似,可能是治疗肥胖相关疾病的一种很有前景的方法。鉴于脂联素或AdipoRon对炎症反应和能量代谢的调节作用,它们可能被赋予治疗组织损伤的潜力。因此,对其作用和可能的机理进行了研究。肝细胞(L02)和巨噬细胞(RAW264.7)的体外研究表明AdipoRon具有保护和抗炎作用。在d-半乳糖胺(D-GalN)诱导的急性肝损伤小鼠中验证了其作用:AdipoRon或双环醇(阳性参比药物)预处理可使肝脏病变恢复,其特点是血清学和肝脏生物标志物(AST、ALT、MDA和nos)显著升高。此外,AdipoRon还能减轻肝脏炎症,表现为促炎巨噬细胞浸润减少,肿瘤坏死因子-α (TNF-α)、转化生长因子-1 (TGF-β1)、白细胞介素-1β (IL-1β)、白细胞介素-6 (IL-6)减少;同时通过磷酸化反过来促进AMPK活化。结合肝脏组织病理学,这些结果证明了AdipoRon对d - galn诱导的损伤具有肝脏保护作用,这可能归因于炎症的衰减,自由基反应的抑制以及肝脏能量代谢的增强。[2] 脂联素(APN)是一种心脏保护分子。它在糖尿病中的减少加重了心肌缺血/再灌注(MI/R)损伤。虽然动物给药APN能减轻心肌梗死/再灌注损伤,但多种因素限制了其临床应用。目前的研究调查了AdipoRon,第一个结合APN受体的口服活性分子,是否可以保护心脏免受MI/R损伤,如果是这样,描述相关机制。野生型(WT)、APN敲除型(APN- ko)和心肌细胞特异性ampk优势阴性(AMPK-DN)小鼠分别给予载药或AdipoRon (50 mg/kg,心肌梗死前10分钟),并进行心肌梗死/心肌缺血再灌注(30 min/3-24 h)。与载药小鼠相比,经DNA阶梯形成、TUNEL染色和caspase-3活化测定,口服AdipoRon可显著改善心肌功能,减轻心肌缺血后细胞凋亡(均P < 0.01)。在APN (APN- ko)或AMPK (AMPK- dn)缺失的小鼠中,MI/ r诱导的凋亡细胞死亡显著增强。在APN-KO小鼠中,AdipoRon减轻MI/R损伤的程度与在WT小鼠中观察到的相同。在AMPK-DN小鼠中,AdipoRon的抗凋亡作用被部分抑制但未丧失。最后,通过降低NADPH氧化酶的表达和超氧化物的产生,AdipoRon显著减轻了缺血后氧化应激。总的来说,这些结果首次证明,口服活性APN受体激活剂AdipoRon可以有效减轻缺血后心脏损伤,支持APN受体激动剂作为治疗肥胖相关疾病(如2型糖尿病)引起的心血管并发症的一种有前景的新治疗方法。[3] 目的:AdipoRon是一种脂联素受体激动剂,最近被提出用于治疗胰岛素抵抗和高血糖。由于脂联素通过no介导的信号传导起到血管保护作用,因此我们假设脂poron类似地发挥潜在的有益血管舒张作用。因此,我们研究了脂肪蛋白是否诱导血管松弛,以及通过什么机制促进血管松弛。 方法:采用压力肌图和钢丝肌图评价大鼠骨骼肌动脉和小鼠脑/冠状动脉血管功能。 结果:采用qPCR方法,在骨骼肌、脑和冠状动脉中检测到脂联素受体的mRNA表达。化合物C (10 μM)不消除脂肪素引起的血管松弛;AMPK抑制剂)。l-NAME/吲哚美辛/apamin/TRAM-34联合抑制内皮依赖性松弛仅能轻微降低脂肪素介导的脑和冠状动脉血管松弛。经ec剥离的冠状动脉对AdipoRon的松弛反应与完整血管相似,表明AdipoRon直接影响VSMCs。从小鼠基底动脉和LAD动脉分离的VSMCs测量的K(+)电流未被AdipoRon改变。在冠状动脉中,AdipoRon诱导血管松弛,但VSMC没有明显降低[Ca(2+)]。脂联素本身在完整的冠状动脉中引起血管扩张,而在ec剥脱的动脉中没有引起明显的扩张,这与脂联素的内皮依赖性一致。 结论:AdipoRon发挥血管舒张作用的机制与脂联素不同。脂肪素诱导的血管松弛的主要机制独立于内皮依赖性松弛因子、AMPK激活、K(+)外排介导的超极化和细胞质[Ca(2+)]i的减少。 |
||
| 酶活实验 |
Binding Assay。[1]
表面等离子体共振测量由BIAcore X100系统(GE Healthcare)和传感器芯片SA进行。用杆状病毒系统表达人AdipoR1和AdipoR2,并纯化至均匀性。然后将AdipoR1和AdipoR2样品重组为含有生物素型磷脂酰乙醇胺的卵磷脂酰胆碱脂质体。小鼠全长脂联素生成如前所述。采用标准固定方案(GE Healthcare),将AdipoR1和AdipoR2固定在传感器芯片SA上,达到2500 - 3000响应单位(RU)的水平。我们以视紫红质受体为对照,观察到AdipoRon确实不与视紫红质受体发生反应。实验采用流动缓冲液(20 mM Hepes, pH 7.4, 200 mM NaCl, 10%甘油,0.05% (v/v)表面活性剂P20),在25℃下进行。使用AdipoRon(0.49-31.25µM)或脂联素(1.5 ng-3.75µg)范围进行结合分析。使用Biacore X100评价软件测定化合物或蛋白质的平衡解离常数(KD)。 3H-labelled AdipoRon结合试验[1] tritium - labeled AdipoRon在下图中星号所示的位置进行了氚标记。在羧酸固体(25 mg)中加入0.5 ml亚硫酰氯,仔细加热悬浮液使固体溶解。混合物在室温下搅拌1小时,用氮气流除去多余的亚硫酰氯,并在真空下抽干残留物30分钟。将未标记的二盐酸胺(35 mg)溶解在水中(1 ml)。加入碳酸钾(50mg),将游离胺提取到二氯甲烷(3ml)中。该有机溶液用无水硫酸钠(5毫克)干燥。悬浮液经过过滤,溶剂通过旋转蒸发除去。将游离胺碱溶于二氯甲烷(2 ml):三乙胺(50 μl)中。将氯酸溶解在二氯甲烷中,并加入到上述胺基溶液中。将混合物搅拌30分钟,使氯酸与胺偶联。用二氧化硅薄层色谱板在CH2Cl2:MeOH:AcOH(95:5:0.1)中洗脱。然后用二氧化硅Sep-Pak (2g)纯化该混合物,用3 × 2ml二氯甲烷洗脱,然后用CH2Cl2:MeOH:AcOH (95:5:0.1) 3 × 2ml洗脱。将馏分3 - 6混合,在真空下除去溶剂过夜,得到无色油。然后对产物进行氚化处理(296 MBq/mmol)。结合实验按照前面描述的方法进行,稍作修改。细胞与含有指定浓度的3h标记的AdipoRon和未标记的竞争对手的结合缓冲液(冰冷的磷酸盐缓冲sarine (PBS))在25℃下孵育1小时。然后用PBS洗涤细胞10次,在0.1 M NaOH, 0.1% SDS中裂解,并用-counter18,19,22测定细胞结合放射性。使用200倍的未标记AdipoRon过量测定非特异性结合。特异性结合是通过从总结合中减去非特异性结合来计算的。 |
||
| 细胞实验 |
细胞与细胞培养[2]
使用永生化的正常人肝细胞L02和小鼠单核细胞系RAW264.7… AdipoRon对肝细胞的体外保护作用[2] 在体外L02细胞株上检测了AdipoRon的肝保护作用,为其活性和机制的研究提供了一些线索。结果表明,5-50 μM AdipoRon预处理可明显以剂量依赖性方式减弱TNF-α和TGF-β1的表达(图2B),而对肝细胞的凋亡或增殖几乎没有变化(图2A),这可能暗示AdipoRon通过抑制促炎作用而具有肝保护作用。 |
||
| 动物实验 |
|
||
| 参考文献 |
|
||
| 其他信息 |
Adiponectin secreted from adipocytes binds to adiponectin receptors AdipoR1 and AdipoR2, and exerts antidiabetic effects via activation of AMPK and PPAR-α pathways, respectively. Levels of adiponectin in plasma are reduced in obesity, which causes insulin resistance and type 2 diabetes. Thus, orally active small molecules that bind to and activate AdipoR1 and AdipoR2 could ameliorate obesity-related diseases such as type 2 diabetes. Here we report the identification of orally active synthetic small-molecule AdipoR agonists. One of these compounds, AdipoR agonist (AdipoRon), bound to both AdipoR1 and AdipoR2 in vitro. AdipoRon showed very similar effects to adiponectin in muscle and liver, such as activation of AMPK and PPAR-α pathways, and ameliorated insulin resistance and glucose intolerance in mice fed a high-fat diet, which was completely obliterated in AdipoR1 and AdipoR2 double-knockout mice. Moreover, AdipoRon ameliorated diabetes of genetically obese rodent model db/db mice, and prolonged the shortened lifespan of db/db mice on a high-fat diet. Thus, orally active AdipoR agonists such as AdipoRon are a promising therapeutic approach for the treatment of obesity-related diseases such as type 2 diabetes.[1]
Adiponectin is an antidiabetic and antiatherogenic adipokine, which plays distinct roles in the balance of energy homoeostasis. As an insulin sensitizing hormone, adiponectin exerts multiple biological effects by the specific receptors (AdipoR1 and AdipoR2), through activation of AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor (PPAR)α pathways. AdipoRon, an orally active synthetic small-molecule AdipoR agonist, shows very similar effects to adiponectin in vitro and in vivo, which could be a promising therapeutic approach for obesity-related disorders. In view of the regulatory effects of adiponectin or AdipoRon on inflammatory response and energy metabolism, they might be endowed a curative potential for tissue damage. Hence, its effects and possible mechanism were investigated. In vitro studies on hepatocytes (L02) and macrophages (RAW264.7) suggested a protective and anti-inflammatory potential of AdipoRon. The effects were verified in acute hepatic injury mice induced by d-galactosamine (D-GalN): hepatic lesions were restored by AdipoRon or bicyclol (positive reference drug) pretreatment, which were characterized by a significant increase in serological and hepatic biomarkers (AST, ALT, MDA and NOSs). Besides, AdipoRon attenuated the inflammation in the liver, characterized by the dwindling proinflammatory macrophage infiltration, as well as the shrinkage of tumor necrosis factor-α (TNF-α), transforming growth factor beta 1 (TGF-β1), interleukin-1 beta (IL-1β) and interleukin-6 (IL-6); meanwhile conversely promoted AMPK activation by phosphorylation. Combined with liver histopathology, these results demonstrated the hepatoprotective effects of AdipoRon against D-GalN-induced damage, which might be ascribed to the attenuation of inflammation, inhibition of free radical reactions, as well as enhancement of liver energy metabolism.[2] Adiponectin (APN) is a cardioprotective molecule. Its reduction in diabetes exacerbates myocardial ischemia/reperfusion (MI/R) injury. Although APN administration in animals attenuates MI/R injury, multiple factors limit its clinical application. The current study investigated whether AdipoRon, the first orally active molecule that binds APN receptors, may protect the heart against MI/R injury, and if so, to delineate the involved mechanisms. Wild-type (WT), APN knockout (APN-KO), and cardiomyocyte specific-AMPK dominant negative (AMPK-DN) mice were treated with vehicle or AdipoRon (50 mg/kg, 10 min prior to MI) and subjected to MI/R (30 min/3-24 h). Compared with vehicle, oral administration of AdipoRon to WT mice significantly improved cardiac function and attenuated postischemic cardiomyocyte apoptosis, determined by DNA ladder formation, TUNEL staining, and caspase-3 activation (all P < 0.01). MI/R-induced apoptotic cell death was significantly enhanced in mice deficient in either APN (APN-KO) or AMPK (AMPK-DN). In APN-KO mice, AdipoRon attenuated MI/R injury to the same degree as observed in WT mice. In AMPK-DN mice, AdipoRon's antiapoptotic action was partially inhibited but not lost. Finally, AdipoRon significantly attenuated postischemic oxidative stress, as evidenced by reduced NADPH oxidase expression and superoxide production. Collectively, these results demonstrate for the first time that AdipoRon, an orally active APN receptor activator, effectively attenuated postischemic cardiac injury, supporting APN receptor agonists as a promising novel therapeutic approach treating cardiovascular complications caused by obesity-related disorders such as type 2 diabetes.[3] Objective: AdipoRon, an adiponectin receptor agonist, was recently proposed for treating insulin resistance and hyperglycemia. As adiponectin is vasoprotective via NO-mediated signaling, it was hypothesized that adipoRon similarly exerts potentially beneficial vasodilator effects. We therefore examined if adipoRon induces vasorelaxation and via what contributing mechanisms. Methods: Vascular function was assessed in skeletal muscle arteries from rats and cerebral/coronary arteries from mice using pressure and wire myography. Results: Using qPCR, mRNA for adiponectin receptors was demonstrated in skeletal muscle, cerebral and coronary arteries. AdipoRon-caused vasorelaxation was not abolished by compound C (10 μM; AMPK inhibitor). Inhibition of endothelium-dependent relaxation with combinations of l-NAME/indomethacin/apamin/TRAM-34 only slightly reduced adipoRon-mediated vasorelaxation in cerebral and coronary arteries. EC-denuded cremaster arteries showed similar relaxant responses to adipoRon as in intact vessels, suggesting adipoRon directly impacts VSMCs. K(+) currents measured in VSMCs isolated from mouse basilar and LAD arteries were not altered by adiopRon. In cremaster arteries, adipoRon induced vasorelaxation without a marked decrease in VSMC [Ca(2+)]i . Adiponectin, itself, caused vasodilation in intact cremaster arteries while failing to cause significant dilation in EC-denuded arteries, consistent with endothelium dependency of adiponectin. Conclusions: AdipoRon exerts vasodilation by mechanisms distinct to adiponectin. The dominant mechanism for adipoRon-induced vasorelaxation occurs independently of endothelium-dependent relaxing factors, AMPK activation, K(+) efflux-mediated hyperpolarization and reductions in cytosolic [Ca(2+)]i .[4] |
| 精确质量 |
464.186
|
|---|---|
| CAS号 |
1781835-20-8
|
| 相关CAS号 |
AdipoRon;924416-43-3
|
| PubChem CID |
78243714
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
58.6
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
582
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
TZVJQEGKRLDTHQ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H28N2O3.ClH/c30-26(28-24-15-17-29(18-16-24)19-21-7-3-1-4-8-21)20-32-25-13-11-23(12-14-25)27(31)22-9-5-2-6-10-22;/h1-14,24H,15-20H2,(H,28,30);1H
|
| 化学名 |
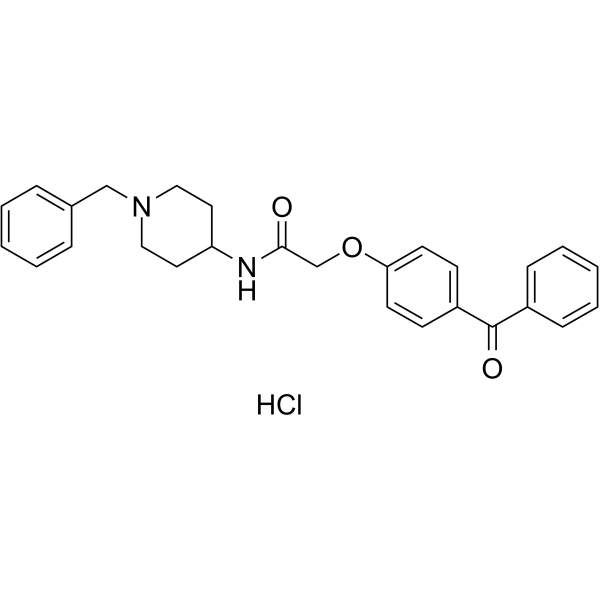
2-(4-benzoylphenoxy)-N-(1-benzylpiperidin-4-yl)acetamide;hydrochloride
|
| 别名 |
AdipoRon hydrochloride; 1781835-20-8; 2-(4-Benzyoylphenoxy)-N-[1-(phenylmethyl)-4-piperidinyl]acetamidehydrochloride; 2-(4-benzoylphenoxy)-N-(1-benzylpiperidin-4-yl)acetamide;hydrochloride; AdipoRon?; 2-(4-Benzyoylphenoxy)-N-[1-(phenylmethyl)-4-piperidinyl]acetamide hydrochloride; C27H28N2O3.HCl; 924416-43-3 (free base);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。