| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5g |
|
||
| Other Sizes |

| 体外研究 (In Vitro) |
在猪中,对羟基苯甲酸丁酯(0-500 μM,44 小时)会抑制受精、卵裂和囊胚形成的速率 [2]。在猪卵母细胞中,对羟基苯甲酸丁酯会导致 DNA 损伤、细胞封闭和自噬 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
13 周内,皮下注射对羟基苯甲酸丁酯(0-50 mg/kg/天)并未引起全身毒性[1]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
By the oral route, parabens are rapidly absorbed, metabolized, and excreted. The metabolic reactions and conversions in mammals vary with the chain length of the ester, the animal species, route of administration, and quantity tested. The metabolism of parabens in humans appears to be most closely related to that of dogs. The rate of metabolite excretion appears to decrease with increasing molecular weight of the ester. /4-Hydroxybenzoates (Parabens)/ After butylparaben is intravenously infused into the dog, nonhydrolyzed butylparaben is found in brain, spleen, and pancreas. In liver, kidney, and muscle, it is immediately hydrolyzed to p-hydroxybenzoic acid. Six hours after oral administration of 1.0 g/kg to dogs, the peak plasma concentration of free and total butyl paraben (15 and 141 ug/cu cm) is reached. After 48 hr, butylparaben is eliminated. Skin penetration of methyl, ethyl, propyl and butyl parabens through excised guinea pig dorsal skin was examined, and effects of the penetration enhancers, l-menthol plus ethanol itself and N-dodecyl-2-pyrrolidone, were observed. Permeability of coefficients of the parabens correlated with n-octanol/water partition coefficients. Addition of 1% l-menthol in 15% ethanol about sixteen times increased the permeability coefficient of methyl paraben, whereas this enhancer decreased that of butyl paraben to about one fifth of the control value. A similar, though weaker, tendency was observed for the effects of 15% ethanol itself. 0.025% suspension of N-dodecyl-2-pyrrolidone increased the permeability coefficient of methyl paraben about seven times, whereas it did not change that of butylparaben significantly. Therefore, dependency of the permeability coefficients of the parabens on n-octanol/water partition coefficients almost disappeared in the presence of this compound. A spin label study with stratum corneum lipid liposomes revealed that increase of fluidity of the lipid bilayer by these penetration enhancers corresponded with their enhancement effects on skin penetration of methyl paraben. Perturbation of stratum corneum lipid lamella thus seems to be related with their enhancement of the absorption of hydrophilic paraben. Intravenous (IV) injections at 50 mg/kg methylparaben, ethylparaben, propylparaben, or butylparaben were administered to groups of three or more fasted dogs. Similarly, these compounds were administered orally at a dose of 1.0 g/kg. Blood and urine were analyzed at predetermined intervals. Immediately following IV injection, very little ester remained in the blood. Metabolites were detectable in the blood up to 6 hr postinjection and 24 hr postingestion. Recovery of all esters but butylparaben ranged from 58 to 94% of the administered dose. Absorption was essentially complete. Recovery of butylparaben after oral administration was 40% and 48 after IV administration. The authors considered this finding a result of less effective hydrolysis of butylparaben. Dogs given 50 mg/kg were then killed and the distribution of esters and metabolites to organs was determined. Pure ester was recovered only in the brain, spleen, and pancreas. High concentrations of metabolites were detected in the liver and kidneys. With in vitro assays, it was found that esterases in the liver and kidneys of the dog were extremely efficient in hydrolyzing parabens --- complete hydrolysis after 3 minutes for all parabens except butylparaben, which took 30 to 60 minutes. No accumulation of parabens was observed in the tissues of dogs given orally 1 g/kg/day methylparaben or propylparaben for 1 year. The rate of urinary excretion of esters and metabolites in these dogs increased to such an extent that after 24 hr, 96 % of the dose was excreted in the urine. This is contrasted with dogs given a single dose of paraben in which the 96 % excretion level was not attained until 48 hr. When 10 % methylparaben or propylparaben in hydrophilic ointment was applied to the skin of a white rabbit for 48 h, esters and metabolites were not detected in the kidneys. For more Absorption, Distribution and Excretion (Complete) data for BUTYLPARABEN (9 total), please visit the HSDB record page. Metabolism / Metabolites In mice, rats, rabbits, or dogs, butyl paraben is excreted in the urine as unchanged benzoate, p-hydroxybenzoic acid, p-hydroxyhippuric acid (p-hydroxybenzoylglycine), ester glucuronides, ether glucuronides, or ether sulfates. By the oral route, parabens are rapidly absorbed, metabolized, and excreted. The metabolic reactions and conversions in mammals vary with the chain length of the ester, the animal species, route of administration, and quantity tested. The metabolism of parabens in humans appears to be most closely related to that of dogs. The rate of metabolite excretion appears to decrease with increasing molecular weight of the ester. /4-Hydroxybenzoates (Parabens)/ The penetration and metabolism of butylparaben using viable, full-thickness human skin /is described/. ... A total of 21% of the radiolabel penetrated to the receptor fluid after 24 hr. ... the principle metabolite, hydroxybenzoic acid, was detected in the receptor fluid, with barely detectable levels of butylparaben and no ethylparaben, in this study of full-thickness skin. ... This work was repeated to again examine the penetration and metabolism of butylparaben (0.4%) in an oil/water emulsion applied to the same full thickness viable human skin ... A finite dose (10 L/cm ) of the 2 emulsion was applied to the skin surface and remained in contact over a 24 hr period without occlusion. (14)C-butylparaben (labeled in the carbon ring) was measured in the receptor fluid. A mean value of 14.9% (+ or - 3.73%) of the radioactive label penetrated the full thickness human skin after 24 hr. The principle metabolite, hydroxybenzoic acid, was found in the receptor fluid (mean of 15.2% + or - 5.23%) of all 10 replications (skin donated from two individuals), but barely detectable levels of the parent butylparaben (mean of 0.225% 0.063%) were found only in 5 of 10 replications. The authors interpreted these results to confirm the near complete first-pass metabolism of butylparaben to p-hydroxybenzoic acid in human skin. ... A study /was conducted/ of the in vitro dermal penetration and metabolism of methylparaben and butylparaben in rat and human skin. For each paraben, an oil in water emulsion with both radiolabeled ( C in the carbon 14 ring) and non-radiolabeled paraben was prepared to a target concentration (0.8% for methylparaben and 0.4% for butylparaben). Skin samples (10 replicates for rat skin and 13 replicates for human skin) were mounted in flow-through diffusion cells. Test emulsions were applied evenly at 10 L/cm , one time, with no occlusion. Samples of the receptor 2 fluid from a single skin were pooled, along with reference standards, were mixed with acetonitrile, filtered, and analyzed for methylparaben, butylparaben, and hydroxybenzoic acid using liquid chromatography coupled with mass spectroscopy. ... For Butylparaben, 52.3% was metabolized to hydroxybenzoic acid, with only 5.5% as unmetabolized butylparaben. Metabolism was different in human skin ... For butylparaben, 32.8% appeared as hydroxybenzoic acid and 49.7% as unmetabolized butylparaben. For more Metabolism/Metabolites (Complete) data for BUTYLPARABEN (9 total), please visit the HSDB record page. Butyl-4-hydroxybenzoate has known human metabolites that include (2S,3S,4S,5R)-6-(4-butoxycarbonylphenoxy)-3,4,5-trihydroxyoxane-2-carboxylic acid. Biological Half-Life BPB was rapidly cleared in hepatocytes from rat (t(1/2) = 3-4 min) and human (t(1/2) = 20-30 min). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: HUMAN EXPOSURE AND TOXICITY: Butylparaben was a skin irritant in man. Experimental studies in volunteers failed to uncover any sensitizing potential, but sensitization to butylparaben has been demonstrated in dermatitis patients. ANIMAL STUDIES: A low acute oral toxicity was seen in mice treated with the ester whereas the sodium salt was of moderate toxicity. In mice, there were effects on the spleen and thymus as well as liver damage. Butylparaben in the diet produced cell proliferation in the forestomach of rats, but it was noncarcinogenic in a mouse chronic feeding study. It was not mutagenic in Ames bacterial tests. In one in vitro study, sperm were not viable at concentrations as low as 1 mg/mL for butylparaben. Epididymis and seminal vesicle weight decreases were reported in rats given a 1% oral butylparaben dose; and decreased sperm number and motile activity in F(1) offspring of rats maternally exposed to 100 mg/kg per day were reported. Decreased sperm numbers and activity were reported in F(1) offspring of female rats given Butylparaben by subcutaneous injection at 100 or 200 mg/kg per day, but there were no abnormalities in the reproductive organs. Butylparaben does bind to estrogen receptors in isolated rat uteri, but with an affinity orders of magnitude less than natural estradiol. ECOTOXICITY STUDIES: The aquatic toxicity on fish, daphnia, and algae was weaker for the parabens with a shorter alkyl chain than those with a longer alkyl chain as predicted by their hydrophobicity. The plasma vitellogenin concentration of male medaka increased for concentrations of 200, 100 ug/L n-butylparaben, i-butylparaben for 14 days. In a rainbow trout Oncorhynchus mykiss increases in average plasma vitellogenin levels were seen at oral exposure to 9 mg butylparaben/kg for 2 days. In other experiment, butylparaben was estrogenic at 10 mg/kg bw in rainbow trout. Interactions The individual and combined (binary mixtures) (anti)androgenic effect of butylparaben (BuPB), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and propyl gallate (PG) was evaluated using the MDA-kb2 cell line. Exposing these cells to AR agonists results in the expression of the reporter gene (encoding for luciferase) and luminescence can be measured in order to monitor the activity of the reporter protein. In case of the evaluation of the anti-androgenic effect, the individual test compounds or binary mixtures were tested in the presence of a fixed concentration of a strong AR agonist (1000 pM 5-alpha-dihydrotestosterone; DHT). Cell viability was assessed using a resazurin based assay. For PG, this is the first report in the literature concerning its (anti)androgenic activity. In case of both individual and mixture testing none of the compounds or binary combinations showed androgenic activity. When tested in the presence of DHT, BuPB, BHA and BHT proved to be weak anti-androgens and this was confirmed during the evaluation of binary mixtures (BuPB+BHA, BuPB+BHT and BHA+BHT). Besides performing the in vitro testing of the binary combinations, two mathematical models (dose addition and response addition) were evaluated in terms of accuracy of prediction of the anti-androgenic effect of the selected binary mixtures. The dose addition model guaranteed a good correlation between the experimental and predicted data. However, no estimation was possible in case of mixtures containing PG, due to the lack of effect of the compound in case of the individual testing. Parabens and phthalates are commercial chemicals widely used in the manufacture of industrial and consumer products frequently found as contaminants in biological fluids. We evaluated the effects of di-(2-ethylhexyl) phthalate (DEHP) (ranging from 10(-9) to 10(-7) m [1-100 nm; 0.39-39 ng/mL ]) and butylparaben (BP) (ranging from 10(-8) to 10(-5) m [10 nm-10 um; 1.9 ng m/L to 1.9 ug/ mL ]), alone and in combination, on isolated mouse preantral follicle and human granulosa cell (hGC) cultures to study direct effects on follicle growth and ovarian steroidogenesis. Our results revealed that, in follicle culture, DEHP and BP attenuate estradiol output but only when present together. DEHP decreases progesterone concentrations in the spent media of hGC cultures, an effect that was attenuated when BP was added together with DEHP. Although changes in steroidogenesis were observed, no effects on follicular development or survival were noted in the culture systems. We suggest that BP and DEHP act with additive effect to decrease estradiol production whereas at later stages of follicle development BP blocks the effect of DEHP in hGCs resulting in decreased progesterone output. Taken together our results suggest that DEHP and BP adversely affect steroidogenesis from the preantral stage onward and the effects of these chemicals are both stage-dependent and modified by co-exposure. To evaluate the estrogenic activities of several chemicals such as 17beta-estradiol (E2), rho-nonylphenol, bisphenol A, butylparaben, and combinations of these chemicals, /the authors/ used recombinant yeasts containing the human estrogen receptor [Saccharomyces cerevisiae ER + LYS 8127]. ... E2 was most active in the recombinant yeast assay, followed by rho-nonylphenol, bisphenol A, /and/ butylparaben. The combinations of some concentrations of 17beta-estradiol as a strong estrogen and bisphenol A or butylparaben as a weak estrogen showed additive estrogenic effects. Also, the combinations of some concentrations of nonlyphenol and butylparaben and combination of butylparaben and bisphenol A showed additive effects in the estrogenic activity. Therefore, the estrogenic activities of the combinations of two chemicals were additive, not synergistic. Exposure to endocrine disrupting chemicals (EDCs) during development can have negative consequences later in life. In this study we investigated the effect of perinatal exposure to mixtures of human relevant EDCs on the female reproductive system. Rat dams were exposed to a mixture of phthalates, pesticides, UV-filters, bisphenol A, butylparaben, as well as paracetamol. The compounds were tested together (Totalmix) or in subgroups with anti-androgenic (AAmix) or estrogenic (Emix) potentials. Paracetamol was tested separately. In pre-pubertal rats, a significant reduction in primordial follicle numbers was seen in AAmix and PM groups, and reduced plasma levels of prolactin was seen in AAmix. In one-year-old animals, the incidence of irregular estrous cycles was higher after Totalmix-exposure and reduced ovary weights were seen in Totalmix, AAmix, and PM groups. These findings resemble premature ovarian insufficiency in humans, and raises concern regarding potential effects of mixtures of EDCs on female reproductive function. Endocrine-disrupting compounds can interfere with the endocrine organs or hormone system and cause tumors, birth defects and developmental disorders in humans. The estrogen-like activity of compounds has been widely studied but little is known concerning their possible modulation of the glucocorticoid receptor. Steroidal (synthetic and natural) and non-steroidal endocrine-active compounds commonly occur as complex mixtures in human environments. Identification of such molecular species, which are responsible for modulating the glucocorticoid receptor are necessary to fully assess their risk. We have used the MDA-kb2 cell line, which expresses endogenous glucocorticoid receptor and a stably transfected luciferase reporter gene construct, to quantify the glucocorticoid-like activity of four compounds present in products in everyday use -propylparaben (PP), butylparaben (BP), diethylhexyl phthalate (DEHP) and tetramethrin (TM). We tested all possible combinations of these compounds at two concentrations (1 uM and 10 nM) and compared their glucocorticoid-like activity. At the concentration of 1 uM seven mixtures were identified to have glucocorticoid-like activity except: DEHP+TM, BP+TM, DEHP+PP+TM, BP+PP+TM. At the concentration of 10 nM only three mixtures have glucocorticoid modulatory activity: DEHP+PP, BP+PP, DEHP+BP+PP+TM. Identified glucocorticoid-like activities were between 1.25 and 1.51 fold at the concentration of 1 uM and between 1.23 and 1.44 fold at the concentration of 10 nM in comparison with the solvent control. Individually BP, PP, and DEHP had glucocorticoid-like activity of 1.60, 1.57 and 1.50 fold over the solvent control at the concentration of 1 uM. On the other hand PP and DEHP, at the concentration of 10nM, showed no glucocorticoid-like activity, while BP showed 1.44 fold. The assertion that individual glucocorticoid-like compounds do not produce harm because they are present at low, ineffective levels in humans may be irrelevant when we include mixed exposures. This study emphasizes that risk assessment of compounds should take mixture effects into account. Non-Human Toxicity Values LD50 Mouse (dd-strain) oral 13200 mg/kg LD50 Mouse oral 5.0 g/kg LD50 Mouse ip 230 mg/kg |
| 参考文献 |
|
| 其他信息 |
N-butyl-p-hydroxybenzoate appears as odorless white crystals or crystalline powder. Tasteless, but numbs the tongue. Aqueous solutions slightly acidic to litmus. (NTP, 1992)
Butylparaben is an organic molecular entity. Butylparaben is a Standardized Chemical Allergen. The physiologic effect of butylparaben is by means of Increased Histamine Release, and Cell-mediated Immunity. Butylparaben has been reported in Strychnos cathayensis and Carica papaya with data available. Butylparaben is a preservative and flavouring agent. Butylparaben has been shown to exhibit anti-microbial function Butylparaben belongs to the family of Hydroxybenzoic Acid Derivatives. These are compounds containing an hydroxybenzoic acid (or a derivative), which is a benzene ring bearing a carboxylic acid. (A3205). See also: Butylparaben; ethylparaben; methylparaben (component of). Therapeutic Uses It is used as a pharyngeal antiseptic in combination with other parabens. |
| 分子式 |
C11H14O3
|
|---|---|
| 分子量 |
194.2271
|
| 精确质量 |
194.094
|
| CAS号 |
94-26-8
|
| 相关CAS号 |
Butylparaben-d4;1219798-67-0;Butylparaben-13C6;1416711-53-9;Butylparaben sodium;36457-20-2;Butylparaben-d9;1216904-65-2
|
| PubChem CID |
7184
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
309.2±15.0 °C at 760 mmHg
|
| 熔点 |
67-70 °C(lit.)
|
| 闪点 |
129.2±13.2 °C
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
| 折射率 |
1.526
|
| LogP |
3.46
|
| tPSA |
46.53
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
171
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
QFOHBWFCKVYLES-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C11H14O3/c1-2-3-8-14-11(13)9-4-6-10(12)7-5-9/h4-7,12H,2-3,8H2,1H3
|
| 化学名 |
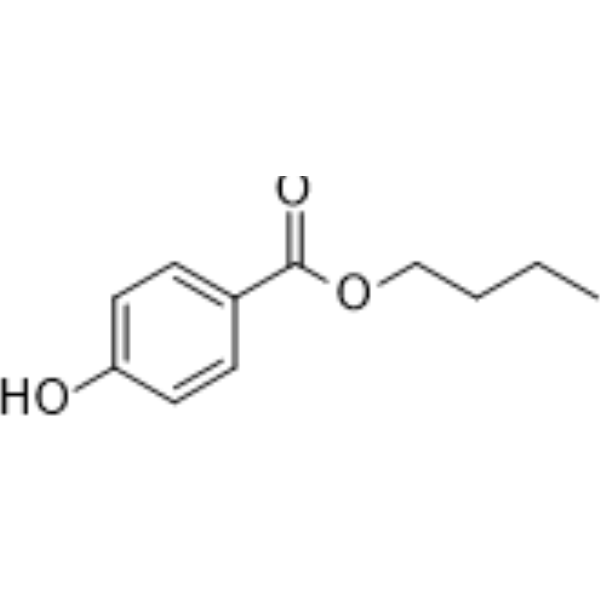
butyl 4-hydroxybenzoate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 2.0 mg/mL (~10.30 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.1485 mL | 25.7427 mL | 51.4854 mL | |
| 5 mM | 1.0297 mL | 5.1485 mL | 10.2971 mL | |
| 10 mM | 0.5149 mL | 2.5743 mL | 5.1485 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。