| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
抗生素头孢泊肟(头孢泊肟酸)对革兰氏阴性厌氧菌(Bacteroidetesceae)的最低抑菌浓度(MIC)为0.125-4 mg/L。观察到头孢泊肟抑制小韦荣球菌的 MIC 值为 0.25-8 mg/L。头孢泊肟可抑制糖酵解链球菌、微小消化链球菌和布氏瘤胃球菌,最低抑制浓度 (MIC) 低于每升 2 毫克 [1]。头孢泊肟或头孢泊肟酸可抑制肺炎链球菌和化脓性链球菌的菌群。菌落形成单位[2]。
|
|---|---|
| 体内研究 (In Vivo) |
在治疗方面,小鼠对头孢菌素(2.5 至 50 mg/kg;口服;每 8 小时一次;连续 48 小时)反应良好 [3]。
|
| 动物实验 |
Animal/Disease Models: Female Swiss CD1 mice [3]
Doses: 2.5, 5, 10, 25, 40 and 50 mg/kg Route of Administration: Oral; Route of Administration: Oral. Every 8 hrs (hrs (hours)); 48 hour Experimental Results: Efficacy value >350 obtained. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration of 100 mg of cefpodoxime proxetil to fasting subjects, approximately 50% of the administered cefpodoxime dose was absorbed systemically. Over the recommended dosing range (100 to 400 mg), approximately 29 to 33% of the administered cefpodoxime dose was excreted unchanged in the urine in 12 hours. Biological Half-Life 2.09 to 2.84 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information indicates that cefpodoxime produces low levels in milk and is not be expected to cause any adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Cefpodoxime is acceptable in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Hyperprolactinemia and bilateral galactorrhea occurred in a nonpregnant, 40-year-old woman taking cefpodoxime 200 mg twice daily for 2 days. Seven days after stopping the drug, galactorrhea ceased and the serum prolactin dropped markedly into the normal range. One month later it had dropped further. Because no other cause could be found, the authors determined that the galactorrhea and hyperprolactinemia were probably caused by cefpodoxime. A 22-year-old woman who had been taking slow-release venlafaxine 150 mg daily for 3 months reported bilateral breast engorgement and galactorrhea for 3 days after being prescribed cefpodoxime 200 mg twice daily for 14 days 2 weeks prior. Laboratory and head CT results were normal except for a slight elevation in alkaline phosphatase and an elevated serum prolactin level. Her galactorrhea began decreasing within 2 weeks and disappeared in 3 weeks with no change in venlafaxine dosage. Her serum prolactin level also returned to normal. The authors felt that her symptoms and hyperprolactinemia were probably caused by cefpodoxime. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 22 to 33% in serum and from 21 to 29% in plasma. |
| 参考文献 |
[1]. Werner H, et, al. Comparative in vitro activity of cefpodoxime against anaerobes other than Bacteroides fragilis. Infection. 1991 Sep-Oct;19(5):377-9.
[2]. Valentini S, et, al. In-vitro evaluation of cefpodoxime. J Antimicrob Chemother. 1994 Mar;33(3):495-508. [3]. Pérez-Trallero E, et, al. Prediction of in-vivo efficacy by in-vitro early bactericidal activity with oral beta-lactams, in a dose-ranging immunocompetent mouse sepsis model, using strains of Streptococcus pneumoniae with decreasing susceptibilities to penicillin. J Chemother. 2001 Apr;13(2):118-25. |
| 其他信息 |
Cefpodoxime is a third-generation cephalosporin antibiotic with methoxymethyl and (2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamino substituents at positions 3 and 7, respectively, of the cephem skeleton. Given by mouth as its proxetil ester prodrug, it is used to treat acute otitis media, pharyngitis, and sinusitis. It has a role as an antibacterial drug. It is a cephalosporin and a carboxylic acid.
Cefpodoxime is an oral third generation cephalosporin antibiotic with effectiveness against most Gram positive and Gram negative bacteria. Commonly used to treat acute otitis media, pharyngitis, and sinusitis, cefpodoxime proxetil is a prodrug which is absorbed and de-esterified by the intestinal mucosa to Cefpodoxime. Cefpodoxime is a Cephalosporin Antibacterial. Cefpodoxime is a third generation semi-synthetic cephalosporin and a beta-lactam antibiotic with bactericidal activity. Cefpodoxime's effect is dependent on its binding to penicillin-binding proteins (PBPs) located in the bacterial cytoplasmic membrane. Binding results in the inhibition of the transpeptidase enzymes, thereby preventing cross-linking of the pentaglycine bridge with the fourth residue of the pentapeptide and interrupting consequent synthesis of peptidoglycan chains. As a result, cefpodoxime inhibits bacterial septum and cell wall synthesis formation. A third-generation cephalosporin antibiotic. Cefpodoxime contains a methoxy group at C-3 of its cephalosporin core. See also: Cefpodoxime Proxetil (active moiety of); Cefpodoxime Sodium (is active moiety of). Drug Indication Indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms. FDA Label Mechanism of Action Cefpodoxime is active against a wide spectrum of Gram-positive and Gram-negative bacteria. Cefpodoxime is stable in the presence of beta-lactamase enzymes. As a result, many organisms resistant to penicillins and cephalosporins, due to their production of beta-lactamase, may be susceptible to cefpodoxime. Cefpodoxime is inactivated by certain extended spectrum beta-lactamases. The bactericidal activity of cefpodoxime results from its inhibition of cell wall synthesis. The active metabolite of cefpodoxime binds preferentially to penicillin binding protein 3, which inhibits production of peptidoglycan, the primary constituent of bacterial cell walls. Pharmacodynamics Cefpodoxime is shown to be effective against most Gram positive and Gram negative bacteria, except Pseudomonas aeruginosa, Enterococcus, and Bacteroides fragilis. |
| 分子式 |
C15H17N5O6S2
|
|---|---|
| 分子量 |
427.46
|
| 精确质量 |
427.062
|
| CAS号 |
80210-62-4
|
| 相关CAS号 |
Cefpodoxime-d3;2477791-28-7
|
| PubChem CID |
6335986
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.8±0.1 g/cm3
|
| 熔点 |
200-202ºC
|
| 折射率 |
1.780
|
| LogP |
0.94
|
| tPSA |
209.98
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
744
|
| 定义原子立体中心数目 |
2
|
| SMILES |
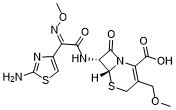
C(C1=C(COC)CS[C@@H]2[C@@H](C(N12)=O)NC(=O)/C(/C1=CSC(N)=N1)=N\OC)(=O)O
|
| InChi Key |
WYUSVOMTXWRGEK-HBWVYFAYSA-N
|
| InChi Code |
InChI=1S/C15H17N5O6S2/c1-25-3-6-4-27-13-9(12(22)20(13)10(6)14(23)24)18-11(21)8(19-26-2)7-5-28-15(16)17-7/h5,9,13H,3-4H2,1-2H3,(H2,16,17)(H,18,21)(H,23,24)/b19-8-/t9-,13-/m1/s1
|
| 化学名 |
5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-3-(methoxymethyl)-8-oxo-, (6R-(6alpha,7beta(Z)))-
|
| 别名 |
U76253AR-3746 U-76253AR3746 R 3763 U 76253AR-3763 R3763
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~584.85 mM)
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3394 mL | 11.6970 mL | 23.3940 mL | |
| 5 mM | 0.4679 mL | 2.3394 mL | 4.6788 mL | |
| 10 mM | 0.2339 mL | 1.1697 mL | 2.3394 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。