| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
RORgamma
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:LYC-55716是Lycera公司开发的一种新型免疫调节药物,是一种合成的口服小分子RAR相关孤儿受体γ(RORγ)激动剂。它将多种抗肿瘤机制结合到单一治疗中,通过调节基因表达来重新编程免疫细胞以改善功能,并减少免疫抑制机制。 2018年1月,Lycera宣布启动一项多中心1B期联合研究,针对晚期、复发或难治性实体瘤(如转移性非小细胞肺癌)患者联合派姆单抗(pembrolizumab)。激酶检测:LYC-55716是Lycera开发的一种新型免疫调节药物,是一种合成的口服小分子RAR相关孤儿受体γ(RORγ)激动剂。它将多种抗肿瘤机制结合到单一治疗中,通过调节基因表达来重新编程免疫细胞以改善功能,并减少免疫抑制机制。细胞检测:LYC-55716选择性地与核受体转录因子RORγ结合,形成受体复合物,易位至细胞核,并与ROR反应元件(RORE)结合,增强17型T细胞的功能、增殖和存活,包括Th17(辅助性 T 细胞)和 Tc17(细胞毒性 T 细胞)可能会增加 T 细胞上共刺激分子的表达并减少共抑制分子的表达,导致 T 细胞产生细胞因子和趋化因子增加,从而减少增殖调节性 T 细胞 (Treg) 的作用,并消除肿瘤诱导的免疫抑制。这最终会诱导 T 细胞介导的针对癌细胞的免疫反应,并导致肿瘤细胞生长减少。 RORγ 是参与 Th17/Tc17 分化的核受体转录因子,在免疫激活中发挥着关键作用。 LYC-55716 具有口服生物利用度,而包括 PD-1/PD-L1 抑制剂在内的新一代免疫肿瘤药物则通过注射给药。
|
| 体内研究 (In Vivo) |
在PK/PD测定中进一步评估了化合物37c/Cintirorgon (LYC-55716) 。RORγt在胸腺中高度表达,调节对胸腺细胞存活和发育重要的基因的表达。RORγ激动剂可以增加胸腺中RORγ靶基因如BclXL和Fbxo27的表达。雌性C57/BL6小鼠服用不同剂量的载体(1%吐温80)或RORγ激动剂Cintirorgon (LYC-55716) (37c),6-16小时后,分离胸腺细胞,制备RNA,并使用Q-PCR检测RORγ靶基因的表达。同时采集样本以测定Cintirorgon (LYC-55716) 的血浆浓度。RORγ靶基因表达的增加与血浆浓度的关系如图3所示,数据拟合得到37c的EC50为338 nM(95%置信区间=202-564 nM)。[2]
在小鼠同基因肿瘤模型中也评估了选择性RORγ激动剂。来源于小鼠肿瘤的细胞系,无论是自发的还是化学诱导的,并重新植入具有免疫能力的小鼠体内,已被用于评估其他免疫疗法。在我们的小鼠结直肠腺癌(MC38)肿瘤模型中,MC38肿瘤细胞被皮下植入雌性C57/BL6小鼠的侧腹。肿瘤植入后3天开始激动剂治疗,每天口服管饲两次。图4显示了用载体或37c(30mg/kg,bid)治疗的荷瘤小鼠的结果。这项研究表明,肿瘤生长抑制率为46%(p<0.05)。该研究又重复了两次,肿瘤生长减少相似(49%和63%,p<0.05)。研究结束时评估肿瘤重量。还观察到肿瘤重量的类似减少。该化合物在所有研究中均具有良好的耐受性,对动物体重没有不良影响。化合物37c和17也抑制了4T1乳腺肿瘤模型中的肿瘤生长。[2] Cintirgon(LYC-55716)是一种RORγ激动剂,口服给药后与核受体转录因子RORγ形成受体复合物。然后,药物转移到细胞核,在那里它与ROR反应元件(RORE)结合以改善功能。17型T细胞,如Th17(辅助T细胞)和Tc17(细胞毒性T细胞),增殖并存活。Th17/Tc17分化由核受体转录因子RORγ促进,RORγ也是免疫激活所必需的。虽然下一代免疫肿瘤药物,如PD-1/PD-L1抑制剂,是通过注射提供的,但口服Cintirorgon (LYC-55716) 也具有生物利用性[1]。 药效学[3] 药效学/靶点结合试验评估了已知RORγ靶基因的mRNA和蛋白质表达:体外刺激后的IL-17A、IL-17F和IL-22(19,20)。RORγ激动剂对这些基因的调节在健康志愿者和癌症患者外周血单核细胞(PBMC)和血液的体外工作中得到证实(22)。如上所述,在第1、2和15天的不同时间点从所有患者身上采集血液样本,并运送到中央实验室进行检测。所有分析物的药效学特征显示,在折叠诱导、细胞因子绝对滴度和PDmax时间方面,患者内部和患者之间存在相当大的差异(见补充表S2和补充图S2中显示的代表性数据)。鉴于RORγ表达和内源性RORγ激动剂的基线水平存在患者特异性差异,观察到的变异性并不出乎意料(补充图S3;参考文献23)。正式的PK/PD分析因每位患者反应幅度的可变性以及血浆浓度低于啮齿动物靶基因调节相关暴露的样本数量不足而变得复杂。然而,药效学数据表明,已经发生了靶向作用,并提供了药效学反应的定性证据,这可以从治疗后细胞因子产生的增加中得到证明(图2A)。此外,32名患者中有31名在至少1次药效学读数中表现出大于2倍的诱导作用,这与预测有效范围内Cintirgon(LYC-55716)暴露水平的药代动力学证据一致。 疗效[3] 在研究的32名患者中,有25名患者的反应可评估。7例因6周内疾病进展(n=4)、患者退出(n=2)和不良事件(n=1)而无法评估。在可评估的患者中,2名(8%)实现了部分缓解,11名(44%)实现了2至12个月的疾病稳定。部分应答者和6名(24%)病情稳定的患者接受了>4个月的Cintirorgon (LYC-55716) 治疗(图3)。一名患有非小细胞肺癌[NSCLC;腺癌;PD-L1肿瘤比例评分(TPS)>50%]的患者在进入研究前接受了pembrolizumab一线治疗(4个周期,首次评估时进展),然后接受了卡铂/培美曲塞一线治疗(3个周期,初次评估时发展),观察到部分反应(图4A)。从治疗开始到最初反应的时间约为6个月;独立的中央放射学审查证实了部分反应。在一名患有肉瘤样乳腺癌症(转移性梭形细胞癌)的患者中发现了第二种部分反应,该患者在研究前接受了卡铂/紫杉醇一线治疗(3个周期,首次评估时进展),然后接受了吉西他滨/多烯紫杉醇一线疗法(6个周期后进展)(图4B)。在该患者中,在肿瘤负荷逐渐减少之后,最初反应的时间为8个月。截至2018年6月,仍有两名患者在接受治疗:一名患有肉瘤样乳腺癌症但有部分反应的患者和一名患有子宫内膜癌症但病情稳定9个月的患者。12名(48%)患者的最佳反应是疾病进展。两名癌症结直肠癌患者在首次评估后,在4个周期结束时因进展而停药,接受irRECIST随访。 |
| 酶活实验 |
ROR激动剂活性的生物测定[2]
使用(i)ROR-配体结合结构域(LBD)TR-FRET测定和(ii)HEK-293T细胞中Gal4-RORγ萤光素酶报告物测定来测试化合物增加RORγ活性的能力。检测程序如下所述 (i) ROR-配体结合结构域TR-FRET测定程序[2] 使用杆状病毒表达系统在SF9细胞中表达HIS标记的ROR-LBD蛋白。将裂解物在测定缓冲液(50 mM Tris pH 7.0、50 mM KCl、1 mM EDTA、0.1 mM DTT、0.01%BSA)中稀释,以获得ROR-384孔测定板中LBD终浓度约为3 nM(需要滴定每批蛋白质)。在测定缓冲液中制备来自辅活化剂SRC1(BiotinCPSSHSSLTERHKILHRLLQEGSPS)的生物素化LXXLL肽储备,并将其加入每个孔中(终浓度为200 nM)。还将铕标记的抗HIS抗体(终浓度0.6 nM)和APC偶联的链霉抗生物素蛋白(终浓度30 nM)的溶液加入到每个孔中。ROR拮抗剂熊果酸也以2µM的终浓度加入。将化合物在DMSO中稀释,并在最终DMSO浓度为1%的测定缓冲液中进一步稀释。所分析的测试化合物的最高浓度为10µM。 [2] 将最终的测定混合物在4°C下孵育过夜或在室温下孵育2小时,并在Envision平板阅读器上测量荧光信号:(激发滤光片=340 nm;APC发射=665 nm;铕发射=615 nm;二向色镜=D400/D630;延迟时间=100µs,积分时间=200µs)。测试化合物的50%有效浓度(EC50)值由665nm的荧光信号除以615nm的荧光信号的商计算得出。在没有熊果酸或测试化合物的情况下,荧光信号的商被设置为100。最大响应被定义为信号中的上平台,通过使用PRISM中的4参数逻辑斯蒂模型进行线拟合来确定。[2] (ii)HEK-293T细胞中Gal4-RORγ萤光素酶报告物测定程序[2] HEK-293细胞的转染在以下方案中,用包含融合到pcDNA3.1neo质粒中RORγ配体结合结构域的Gal4 DNA结合结构域(Gal4 RORγ-LBD)的构建体以及包含pGL4.31 Gal4萤光素酶的报告构建体转染HEK-293细胞。使用空的pcDNA3.1neo和pGL4.31载体类似地制备对照细胞。将室温下的反式-IT试剂(60µL)逐滴加入OptiMEM(Invitrogen,1.5ml)中。通过倒置混合该试剂混合物,然后在室温下孵育5至30分钟。然后将其加入两种表达载体(每种5µg)的溶液中,混合,并在室温下孵育约20分钟。通过移除培养基、用TrypLE Express处理并孵育直至细胞从培养瓶底部分离(约2-5分钟),从培养瓶中收获HEK-293细胞。通过离心收集1000万个细胞,并将其重新悬浮在10毫升的Dulbecco改良Eagle培养基中,该培养基含有10%胎牛血清和100国际单位的青霉素和链霉素。将重新悬浮的细胞和S22转染混合物加入T75烧瓶中,混合并在37°C和5%CO2下孵育过夜。 |
| 细胞实验 |
RORγ活性的细胞测定[2]
如上所述收获细胞,计数并离心以获得所需数量的细胞,然后以0.75 x 106个细胞/mL的浓度重新悬浮在完全生长培养基中。将RORγ拮抗剂熊果酸以2µM的终浓度加入细胞中。将细胞以20µL的细胞悬浮液/孔(10000-15000个细胞/孔)铺在经过白色组织培养处理的384孔板上。将测试化合物以10mM溶解在DMSO中,然后稀释到完全生长培养基中,达到最终预期测试浓度的5倍。将这些5µL/孔的药物储备溶液加入组织培养板中。DMSO的最终浓度为0.2%。将平板短暂离心,然后在37°C和5%CO2下孵育过夜。为了进行测定,使组织培养板平衡至室温,并加入一种Glo萤光素酶试剂(5µL/孔)。将平板短暂离心,然后在室温下孵育10分钟。萤光素酶强度在Envision平板读数器 读取。相对于对照组测定RORγ活性,并使用PRISM绘制试验化合物浓度的函数图,以确定50%有效浓度(EC50)。在没有熊果酸或测试化合物的情况下,萤光素酶信号定义为100。最大响应是使用PRISM中的4参数逻辑模型通过线拟合确定的信号的上平台。 人全血检测方法[2] 简而言之,将人全血收集到肝素钠管中,并加入T细胞活化剂。加入化合物,在37°C和5%CO2的条件下孵育样品18至22小时。通过Trizol/氯仿和RNeasy试剂盒纯化RNA,然后用于RT-PCR和qPCR。S23化合物10、3、1、0.3、0.1、0.03、0µM的稀释范围测试程序分析和T细胞刺激条件在肝素钠管(50 mL)中收集新鲜血液在3种条件下将T细胞活化剂与血液混合:活化剂-1:Dynabeads人T活化剂CD3/CD28,终浓度为15µL/mL;IL-1β和IL23的终浓度为25ng/mL; 活化剂-2:以1µg/mL的终浓度加入可溶性抗CD3和抗CD28终浓度,以10 ng/mL的终浓度添加IL-1β和IL-23。活化剂-3:以10 ngg/mL的终剂量加入PMA,以1µg/mL的终剂量添加Ionomycin。向5 mL活化血液中加入5µL 1000倍的药物储备或DMSO。DMSO的最终浓度为0.1%。在12孔组织培养板上每孔取1.5 mL血液,一式三份在37°C下用5%的二氧化碳孵育18至22小时。 |
| 动物实验 |
Pharmacodynamic Assay [2]
Retinoic acid receptor-related orphan receptor gamma (RORγ) is a transcription factor associated with thymocyte differentiation and maturation, as well as with Type 17 T cell differentiation and function. Activation of RORγ enhances thymocyte survival and induces a transcriptional program which drives Type 17 immune responses and decreases immune suppressive mechanisms. Small molecule agonists of RORγ modulate the expression of target genes including those involved in pro-survival pathways in thymocytes and release of cytokines and chemokines by mature immune cells. In the study, female C57BL/6 mice were given single oral doses of Cintirorgon (LYC-55716) ranging from 3 to 100 mg/kg and euthanized after 6, 10, or 16 hours (n of 4, 5, or 10 animals/time point or dose group). Thymi were collected as a tissue harboring high percentage of RORγ+ cells, RNA was extracted and expression of select RORγ target genes (Fbxo27, Xkrx, ReverbA, BclXL) was S26 analyzed via real-time quantitative polymerase chain reaction (qPCR) to determine the pharmacodynamic (PD) effects of Cintirorgon (LYC-55716) . Plasma samples were also collected at the same time thymi were taken and Cintirorgon (LYC-55716) concentration was determined. Fbxo27 showed the most consistent and robust window of induction following Cintirorgon (LYC-55716) treatment, and thus was used in determining the half-maximal effective concentration (EC50) of Cintirorgon (LYC-55716) . Based on Fbxo27 induction, the EC50 of LYC 55716 is 204 ng/mL (338 nM). Syngeneic Tumor Models [2] MC38 murine colon carcinoma cells or 4T1 murine breast carcinoma cells were implanted subcutaneously into the flank of C57/BL6 or Balb/c mice, respectively. Three days after implantation, mice were dosed with vehicle (1% Tween 80) or test compound at doses noted in text twice a day. Tumor volume, measurable 10–12 days after implantation, was assessed two to three times weekly using caliper measurement of length and width of tumor. Tumor volume calculation = 0.5 x (length x (width)2 ). SCID.beige mice were also used as host mice for MC38 tumor cells to determine the immune system dependence. Mice were taken down after tumor volume reached ethical end point of 2,000 mm3 or at 24 day |
| 药代性质 (ADME/PK) |
Pharmacokinetics [3]
In this study, robust pharmacokinetic sampling was performed on several days during cycle 1. At steady state (day 29), exposures increased linearly from the lowest dose of 150 mg BID through the highest dose of 450 mg BID with exposures overlapping at the 150 and 300 mg BID doses (Supplementary Table S1). There were sufficient terminal phase concentrations from the QD cohorts to determine that Cintirorgon (LYC-55716) Modulation of RORγ-dependent gene expression in murine thymus was used as a preclinical PK/PD model to define 50% and 90% effective concentrations (EC50 and EC90) of 204 and 1777 ng/mL, respectively (data on file). Across cohorts, 100% of patients with PK data available at day 29 (n = 27) had a Cmax that exceeded the EC50 and 93% exceeded the EC90. At the Cmin on day 29, 93% of patients exceeded the EC50 and 44% exceeded the EC90. At 450 mg BID (Cohort 5), the median minimum plasma drug concentration (Cmin) exceeded the EC50 by approximately 22-fold and exceeded the EC90 by 2.5-fold. Twice-daily dosing resulted in minimum plasma concentrations that were consistently higher than once-daily dosing, providing better coverage of the EC50 and EC90 targets (Fig. 2B). Further characterization of Cintirorgon (LYC-55716) 10 μM for both). It was negative for genotoxicity, and the IC20 was >50 μM in a patch clamp assay for effects on the human ether-a-go-go related potassium channel (hERG). It exhibited no inhibition of major Cyp isoforms (2D6, 3A4, 2C19), excellent metabolic stability and moderate plasma protein binding (mouse 96.7% bound; human 98.6% bound). Single-dose pharmacokinetic studies of 37c as its sodium salt were conducted in male and female Sprague Dawley rats (1 mg/kg iv, 30, 100, and 300 mg/kg male PO, 100 mg/kg female PO). Oral bioavailability was high (≥100%). Following an IV dose, the half-life was 2.8 h, the clearance was high (2.8 h, 48 mL/min/kg) and the Vd was high (5.7 L/kg). Single-dose pharmacokinetic studies of 37c as its sodium salt also were conducted in male cynomolgus monkeys (1 mg/kg iv, 10, 30, 100 PO). Oral bioavailability was good (≥100%), the half-life was 6.6 h, and the clearance was moderate (6.6 h, 7.49 mL/min/kg). The Vd was moderate (1.42 L/kg). The AUCs of single-dose and multiday studies were similar. These studies provided confidence to proceed into GLP toxicology studies and ultimately to select LYC-55716 (Cintirorgon, 37c) as a candidate for clinical development.[2] |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety and tolerability [2]
LYC-55716 was generally well tolerated, with most treatment-related AEs occurring at a grade 1–2 severity level (Table 2). No Grade 4 treatment-related AEs occurred. Grade 3 treatment-related AEs included anemia (n = 2, beginning at weeks 7 and 10), elevated gamma-glutamyl transferase (n = 1, beginning at week 3), and hypophosphatemia (n = 1, 3 separate events beginning at week 4). No patients required dose reductions during the study. A total of 6 patients had dose interruptions, but none were because of treatment-related AEs. Two patients in Cohort 4a discontinued the study because of AEs: 1 patient discontinued because of fatigue (Grade 2; likely treatment related) and weight loss (Grade 2; unrelated to treatment), and 1 patient discontinued because of disease progression that was entered as “discontinued because of AE.” |
| 参考文献 |
|
| 其他信息 |
Cintirorgon is an orally bioavailable agonist of retinoic acid-related orphan receptor gamma (RORg), with potential immunomodulatory and antineoplastic activities. Upon oral administration of cintirorgon, this agent selectively binds to the nuclear receptor transcription factor RORg, forming a receptor complex that translocates to the nucleus, and binds to ROR response elements (ROREs), enhancing the function, proliferation and survival of type 17 T-cells, including Th17 (helper T-cells) and Tc17 (cytotoxic T-cells). This may increase the expression of co-stimulatory molecules and decrease the expression of co-inhibitory molecules on T-cells leading to increased production of cytokines and chemokines by T-cells, decreased proliferation of regulatory T-cells (Tregs), and abrogation of tumor-induced immunosuppression. This ultimately induces a T-cell-mediated immune response against cancer cells and leads to a reduction in tumor cell growth. RORg, the nuclear receptor transcription factor that is involved in Th17/Tc17 differentiation, plays a key role in immune activation. CINTIRORGON is a small molecule drug with a maximum clinical trial phase of I (across all indications) and has 2 investigational indications.
NEW YORK and ANN ARBOR, Mich., Jan. 4, 2017 /PRNewswire/ -- Lycera Corp., a privately held biopharmaceutical company developing breakthrough immune modulatory medicines, announced today the initiation of a Phase 1/2a clinical trial of the Company's novel immuno-oncology therapeutic candidate LYC-55716, in patients with advanced, relapsed, or refractory solid tumors. "We continue to make rapid and significant progress in the development of our novel immune modulators. This is Lycera's third clinical trial initiated in the past 12 months and our first immuno-oncology compound to enter the clinic," said Paul Sekhri, President and CEO of Lycera. "The promising results of our preclinical program have provided confirmation that LYC-55716 modulates gene expression of RORgamma expressing T lymphocyte immune cells, resulting in enhanced effector function, as well as decreased immunosuppression, resulting in decreased tumor growth, and improved survival in in vivo preclinical models. This process of reprogramming immune cells is unique from other currently approved immunotherapies, and based on this, as well as the ability to deliver this agent orally, we believe LYC-55716 could be a significant advancement for patients." "Unlike many immunotherapies that either stimulate the immune system or reduce immune suppression, Lycera's RORgamma agonist has demonstrated in preclinical models that it can simultaneously enhance T-cell function and reduce mechanisms of the immune suppression. Therapy with an oral RORgamma agonist may be able to demonstrate single agent activity, as well as show synergy in combination with other immunotherapies," said John Nemunaitis, a principal investigator and Director of the Mary Crowley Medical Research Center, Dallas, TX. "We are excited to be working with Lycera and to be working on a compound with such a novel mechanism of action." The ARGON trial (Trial of RORgamma Agonist LYC-55716 in Advanced Cancer) is a Phase 1/2a study of LYC-55716 in patients with advanced, relapsed or refractory solid tumors. The initial Phase 1 portion of the study is designed to find the biologically active or maximum tolerated dose of LYC-55716. The study will utilize a 3+3 study design, in which LYC-55716 will be administered orally in subjects with relapsed or refractory solid tumors. The primary endpoints are safety and tolerability, and the study is designed to determine the maximum tolerated dose (MTD) and the recommended Phase 2 dose. Upon dose determination, LYC-55716 will enter Phase 2a, which is expected to enroll approximately 40 patients. The primary efficacy endpoint of the Phase 2a portion of the study will be objective response rate according to response evaluation criteria in solid tumors. About LYC-55716 LYC-55716 is a first in class oral, selective RORgamma agonist. The retinoic acid-related orphan receptor gamma (RORgamma) is a nuclear receptor transcription factor that acts as an immune cell master control switch. RORgamma agonists modulate gene expression to reprogram immune cells for improved function, as well as decrease immunosuppressive mechanisms, resulting in decreased tumor growth and enhanced survival in in vivo preclinical models of cancer. Essentially, Lycera's RORgamma agonist approach "removes the brake" and "pushes on the accelerator" of immune function. [1] Retinoic acid receptor-related orphan receptor γ (RORc, RORγ, or NR1F3) is the nuclear receptor master transcription factor that drives the function and development of IL-17-producing T helper cells (Th17), cytotoxic T cells (Tc17), and subsets of innate lymphoid cells. Activation of RORγ+ T cells in the tumor microenvironment is hypothesized to render immune infiltrates more effective at countering tumor growth. To test this hypothesis, a family of benzoxazines was optimized to provide LYC-55716 (37c), a potent, selective, and orally bioavailable small-molecule RORγ agonist. LYC-55716 decreases tumor growth and enhances survival in preclinical tumor models and was nominated as a clinical development candidate for evaluation in patients with solid tumors. [2] Purpose: Transcription factor retinoic acid receptor-related orphan receptor γ (RORγ) regulates type 17 effector T-cell differentiation and function and is key to immune cell regulation. Synthetic RORγ agonists modulate immune cell gene expression to increase effector T-cell activity and decrease immune suppression. A phase 1 study evaluated the safety and tolerability of LYC-55716 (cintirorgon), a first-in-class, oral, small-molecule RORγ agonist in adults with relapsed/refractory metastatic cancer. Patients and methods: Patients received 28-day treatment cycles of oral LYC-55716; dose and dosing regimen were determined according to pharmacokinetic profile and safety. Primary endpoints were safety and tolerability. Secondary endpoints included pharmacokinetics and objective tumor response rate. Results: No dose-limiting toxicities occurred among the 32 enrolled patients who received LYC-55716 150 mg BID to 450 mg BID. Treatment-related adverse events (AE) were primarily grade 1-2 and included diarrhea (n = 11), fatigue (n = 7), anemia (n = 4), decreased appetite (n = 4), and nausea (n = 4). Grade 3 AEs were anemia (n = 2), elevated gamma-glutamyl transferase (n = 1), and hypophosphatemia (n = 1). Pharmacokinetic concentrations achieved levels expected for target gene regulation. Pharmacodynamic results indicated RORγ pathway engagement. Two patients (NSCLC and sarcomatoid breast cancer) had confirmed partial responses; 11 had disease stabilization for 2 to 12 months (6 received >4 months of treatment). Conclusions: These data support the safety and tolerability of LYC-55716 and selection of 450 mg BID dose for a phase 2a study assessing LYC-55716 clinical activity, safety, and biomarkers in patients with NSCLC, head and neck, gastroesophageal, renal cell, urothelial, and ovarian cancers. [3] |
| 分子式 |
C27H22F6NNAO6S
|
|---|---|
| 分子量 |
625.5119
|
| 精确质量 |
625.096
|
| 元素分析 |
C, 51.84; H, 3.55; F, 18.22; N, 2.24; Na, 3.68; O, 15.35; S, 5.13
|
| CAS号 |
2055538-47-9
|
| 相关CAS号 |
2055538-47-9 (sodium);2055536-64-4 (free acid);
|
| PubChem CID |
134694329
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
104
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
1030
|
| 定义原子立体中心数目 |
1
|
| SMILES |
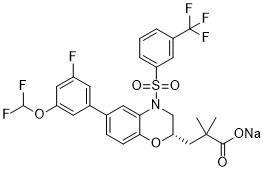
S(C1=C([H])C([H])=C([H])C(C(F)(F)F)=C1[H])(N1C2C([H])=C(C3C([H])=C(C([H])=C(C=3[H])OC([H])(F)F)F)C([H])=C([H])C=2O[C@]([H])(C1([H])[H])C([H])([H])C(C(=O)[O-])(C([H])([H])[H])C([H])([H])[H])(=O)=O.[Na+]
|
| InChi Key |
NWMACPZNQFABGF-BDQAORGHSA-M
|
| InChi Code |
InChI=1S/C27H23F6NO6S.Na/c1-26(2,24(35)36)13-20-14-34(41(37,38)21-5-3-4-17(11-21)27(31,32)33)22-10-15(6-7-23(22)39-20)16-8-18(28)12-19(9-16)40-25(29)30/h3-12,20,25H,13-14H2,1-2H3,(H,35,36)/q+1/p-1/t20-/m0./s1
|
| 化学名 |
sodium
(S)-3-(6-(3-(difluoromethoxy)-5-fluorophenyl)-4-((3-(trifluoromethyl)phenyl)sulfonyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-2-yl)-2,2-dimethylpropanoate
|
| 别名 |
LYC 55716;Cintirorgon sodium; 2055538-47-9; sodium;3-[(2S)-6-[3-(difluoromethoxy)-5-fluorophenyl]-4-[3-(trifluoromethyl)phenyl]sulfonyl-2,3-dihydro-1,4-benzoxazin-2-yl]-2,2-dimethylpropanoate; T6S6P87405; Cintirorgon Sodium?; LYC-55716 SODIUM; UNII-T6S6P87405; NWMACPZNQFABGF-BDQAORGHSA-M; Cintirorgon sodium; LYC-55716 sodium salt; LYC55716
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5987 mL | 7.9935 mL | 15.9870 mL | |
| 5 mM | 0.3197 mL | 1.5987 mL | 3.1974 mL | |
| 10 mM | 0.1599 mL | 0.7993 mL | 1.5987 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。