| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
JAK3
|
|---|---|
| 体外研究 (In Vitro) |
通过基于已建立的JAK3抑制剂NC1153的化学支架的一系列构效关系(SAR)研究,我们鉴定出一种新型、稳定、选择性和有效的JAK3抑制因子,命名为EP009(图1a,补充图S1)。体外激酶测定显示,EP009是JAK3的低微摩尔抑制剂(图1b),对92种人类激酶的脱靶作用有限(补充表S1)。IL-2依赖性T细胞系Kit225和IL-3依赖性前B细胞系BaF/3分别用于测试EP009对JAK3和JAK2的特异性。在Kit225细胞中,EP009降低了IL-2介导的JAK3酪氨酸磷酸化,细胞IC50在10至20μM之间(图1c,上图)。相比之下,在高达50μM的BaF/3细胞中,EP009对IL-3诱导的JAK2酪氨酸磷酸化没有可检测的影响(图1c,下图)。此外,EP009处理降低了Kit225细胞的存活率,72小时的LD50为5.0μM,而未检测到对BaF/3细胞存活率的影响(图1d)。SAR研究表明,环十二酮环的C12亚甲基缺失或C2羟甲基取代显著降低了EP009的疗效(补充图S2)。在相同的实验条件下,JAK抑制剂托法替尼(CP-690550)和鲁索利替尼(INCB-18424)降低了Kit225和BaF/3细胞的存活率(补充图S3)。因此,尽管IC50值表明EP009不如其他JAK抑制剂有效,但激酶选择性的程度使其与通常从ATP竞争性抑制剂中获得的不同。正在进行的研究试图通过应用生化、生物物理和结构研究来阐明EP009抑制JAK3的潜在机制。[1]
|
| 体内研究 (In Vivo) |
JAK3/STAT3活化的减少与细胞存活率的丧失有关,这归因于凋亡的诱导,如胱天蛋白酶3活化和PARP切割的增加所证实的
划译
为了进一步探索EP009介导的JAK3抑制的功能后果和体内活性,我们利用了人类T-NHL ALCL模型系统。EP009以剂量依赖的方式抑制SU-DHL-1细胞中JAK3/STAT3的组成型酪氨酸磷酸化和激活(图2a和2b)。JAK3/STAT3活化的减少与细胞活力的丧失有关,这归因于凋亡的诱导,如胱天蛋白酶3活化和PARP切割的增加所证实的(补充图S5a和5b)。此外,EP009细胞毒性与JNK1/2的激活相关,但与p38介导的应激信号传导和SU-DHL-1中p70 S6激酶介导的生长和存活信号传导的失活无关(补充图S5c、5d和5e)。这些发现强调了通过JAK3抑制促进T-NHL细胞凋亡产生应激的重要性[1]。
|
| 动物实验 |
Thus, tumor xenografts should be exposed to inhibitory concentrations of EP009 previously observed in the cell-based studies. Whether repetitive dosing results in greater tissue accumulation and EP009 concentrations remains to be determined. The therapeutic efficacy of EP009 was further evaluated using a SCID/NOD murine xenograft model of human T-NHL ALCL. Treatment of mice bearing SU-DHL-1 tumors with EP009 given orally at 100 or 200 mg/kg resulted in significant tumor inhibition (>50% reduction; p<0.01) versus placebo control groups (Fig. 2d). Tumor responses were evident three weeks post EP009 treatment suggesting greater tissue accumulation is required for inhibiting tumor cell growth. However, once initiated, tumor responses were maintained for the duration of the treatment. Consistent with the in vitro results, EP009 treatment was associated with reduced levels of Tyr705-phosphorylated STAT3 (p<0.01) compared to vehicle treated control tumors (Fig. 2e and 2f). Considering the effects of EP009 on ALCL cells in vitro and in vivo, these results suggest that constitutive activation of JAK3 represents a viable therapeutic target to treat malignant T-cell lymphoma. Indeed, supporting evidence indicates that JAK3 is a secondary oncogenic driver in ALCL that is induced by autocrine cytokine signaling mechanisms via IL-9 and IL-21. Therefore, EP009 mediated inhibition of JAK3 would serve as a logical therapeutic strategy for intervention in hematopoietic malignancies in primary response to JAK3 activating alleles, and secondary responses where JAK3 acts as an oncogenic driver, such as ALK-positive T-NHL ALCL.[1]
|
| 药代性质 (ADME/PK) |
To assess the clinical potential of EP009, its bioavailability was determined following oral administration (200 mg/kg dosing) to Sprague Dawley rats. Pharmacokinetic analysis showed rapid bioavailability with peak blood concentrations (~6 μM) at 30 min and was detectable up to eight hour.[1]
|
| 参考文献 |
[1]. Leukemia. 2014 Apr;28(4):941-4. |
| 其他信息 |
In conclusion, EP009 is a selective and orally active JAK3 inhibitor that displays a favorable cytotoxicity profile with therapeutic efficacy against JAK3-driven tumor T-cells in vitro, in vivo, and ex vivo. Current cancer drug development is focused on targeted therapies which selectively uncouple cell signaling pathways required for tumor cell growth and survival. The data generated and described in this study support the role of JAK3 as a viable and relevant molecular target in the treatment of T-cell malignancies where compounds such as EP009 can be effective anti-neoplastic agents.
|
| 分子式 |
C14H24O2
|
|---|---|
| 精确质量 |
224.177
|
| 元素分析 |
C, 74.95; H, 10.78; O, 14.26
|
| CAS号 |
1462951-30-9
|
| PubChem CID |
89825463
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
4.7
|
| tPSA |
37.3
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
233
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
VHYYPGIKGAKBQI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H24O2/c1-12-9-7-5-3-2-4-6-8-10-13(11-15)14(12)16/h13,15H,1-11H2
|
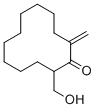
| 化学名 |
2-(hydroxymethyl)-12-methylidenecyclododecan-1-one
|
| 别名 |
EP009; EP-009; EP009; 1462951-30-9; 2-(Hydroxymethyl)-12-methylenecyclododecan-1-one; starbld0004057; SCHEMBL15286031; EP 009
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。