| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
HCV nonstructural 5B protein (NS5B); RNA-dependent RNA polymerase (RdRp)
|
|---|---|
| 体外研究 (In Vitro) |
Filibuvir(0.01-10000 nM;48 h)对 WT 1b 复制子具有剂量依赖性抑制作用,在携带 HCV 复制子的 Huh7.5 细胞中 EC50 约为 70 nM。 HCV 聚合酶和 filibuvir 的解离常数为 29 nM[2]。
Filibuvir 更优先通过延长抑制而不是从头起始来抑制 RNA 合成。 filibuvir 的 IC50 为 73 nM,可减少 PE46 的引物延伸,但对从头启动的 RNA 合成没有明显影响 (IC50=∼5 μM)[2]。 |
| 体内研究 (In Vivo) |
慢性丙型肝炎病毒(HCV)感染需要更有效和耐受性更好的治疗方法。在正在开发的直接作用抗hcv药物中,非结构5B蛋白(NS5B聚合酶)非核苷抑制剂菲布韦。我们在两项1b期临床研究中研究了多剂量filibuvir/非利布韦在未接受治疗和有治疗经验的慢性HCV基因型1感染患者中的抗病毒活性、药代动力学、安全性和耐受性(研究1是一项随机、安慰剂对照剂量递增研究,研究2是一项非随机、开放标签研究)。评估的非布韦剂量范围为每天200- 1400mg,给药时间范围为3-10天。还评估了短期非布韦治疗后NS5B核苷酸序列的基因型变化。非利布韦以剂量依赖性的方式有效抑制病毒复制。平均最大HCV RNA从基线变化范围为-0.97 log(10) IU/mL,非利布韦100mg,每天两次,非利布韦700mg,每天两次,首次治疗的患者为-2.30 log(10) IU/mL。在治疗经验丰富的患者中,每天两次给予450 mg非布韦,HCV RNA降低2.20 log(10) IU/mL。在两项研究中,非利布韦耐受性良好。不良事件的严重程度为轻度或中度。无停药、严重不良事件或死亡报告。NS5B测序鉴定残基423为非利布韦给药后的主要突变位点。
结论:非利布韦在HCV基因型感染患者耐受的剂量下显著降低了HCV RNA浓度。菲利布韦目前正在评估与聚乙二醇化干扰素α 2a加利巴韦林联合治疗初治患者。[1]
|
| 酶活实验 |
表面等离子体共振。[2]
所有分析物结合实验均在Biacore T100仪器上进行,使用n -羟基琥珀酰亚胺酯和1-乙基-3(3-二氨基丙基)盐酸碳二亚胺活化的预处理CM5传感器芯片。1bΔ21 NS5B蛋白及其变体在注射5分钟后固定到约9,000反应单位的标称密度。将化合物注入含有25 mM HEPES (pH 7.4)、10 mM MgCl2、150 mM NaCl、0.01% Tween 20、0.05% β-巯基乙醇和5% DMSO的缓冲液中。所有化合物均表现出1:1的饱和结合行为。为了竞争结合,实验设计包括注射饱和浓度的第一种分析物(160 nM filibuvir),然后立即注射等摩尔比例的分析物混合物(160 nM filibuvir+ 160 nM VX-222或ANA-598)。 |
| 参考文献 |
|
| 其他信息 |
Filibuvir is a member of triazolopyrimidines.
Filibuvir has been used in trials studying the treatment of Hepatitis, Hepatitis C, and Chronic Hepatitis C. Filibuvir is a non-nucleoside polymerase inhibitor of the hepatitis C virus NS5B RNA-dependent RNA polymerase. Filibuvir binds to the non-catalytic Thumb 2 site on viral polymerase and causes a decrease in viral RNA synthesis. Researchers characterized the interaction of filibuvir and VX-222 with the thumb II pocket of the HCV polymerase and determined their effects on 1b/Con1 replicons as well as the polymerase activity. Both filibuvir and VX-222 have binding affinities (Kds) for the HCV polymerase in the nanomolar range, with rapid on rates and relatively slow off rates. In the crystal structure of the filibuvir-polymerase complex, L419 and I482 interact with the pyridine group, while M423 interacts with the cyclopentyl portion of the lactone. Our results show that the latter interaction is more important for stable binding by filibuvir. For VX-222, the crystal structure of the complex is not known, but all three residues we examined contributed to interaction with VX-222. We note that L412, M423, and I482 all help to form a hydrophobic surface within the thumb II domain, and it is likely that they interact hydrophobically with VX-222, likely with the 4-methy-cyclohexanoyl group. Studies with inhibitors have demonstrated that the thumb subdomain has an important role in regulating the mode of RNA synthesis. Benzimidazole-based compounds that bind to the thumb I pocket likely affect the interaction between the Δ1 loop and the thumb subdomain to prevent de novo-initiated RNA synthesis. Chinnaswamy et al. previously showed that a substitution in the thumb I subdomain can affect the interaction between subunits of the polymerase that are needed for de novo-initiated RNA synthesis. The results here with filibuvir and VX-222 and those of Le Pogam et al. with thiophene-based compounds consistently reveal an inhibitory effect on the ability of the HCV polymerase to extend from a primed template. It is quite likely that the thumb region of the polymerase is involved in conformational changes and/or oligomerization states of the HCV polymerase that could regulate the activities of the polymerase, as proposed by Wang et al. Furthermore, VX-222 and filibuvir likely interfere with the required conformational changes required for elongative RNA synthesis. DSF results did show a significant change in polymerase stability indicative of a conformational change (Fig. 4), but additional structural and functional analyses are needed to define the polymerase conformation(s) that could facilitate de novo-initiated or elongative RNA synthesis.[2] |
| 分子式 |
C29H37N5O3
|
|---|---|
| 分子量 |
503.647
|
| 精确质量 |
503.29
|
| 元素分析 |
C, 69.16; H, 7.41; N, 13.91; O, 9.53
|
| CAS号 |
877130-28-4
|
| PubChem CID |
54708673
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.29g/cm3
|
| LogP |
5.124
|
| tPSA |
102.5
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
833
|
| 定义原子立体中心数目 |
1
|
| SMILES |
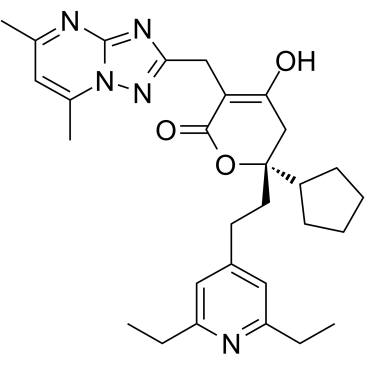
C([C@]1(OC(=O)C(CC2N=C3N=C(C=C(N3N=2)C)C)=C(O)C1)C1CCCC1)CC1C=C(CC)N=C(CC)C=1
|
| InChi Key |
SLVAPEZTBDBAPI-GDLZYMKVSA-N
|
| InChi Code |
InChI=1S/C29H37N5O3/c1-5-22-14-20(15-23(6-2)31-22)11-12-29(21-9-7-8-10-21)17-25(35)24(27(36)37-29)16-26-32-28-30-18(3)13-19(4)34(28)33-26/h13-15,21,35H,5-12,16-17H2,1-4H3/t29-/m1/s1
|
| 化学名 |
(2R)-2-cyclopentyl-2-[2-(2,6-diethylpyridin-4-yl)ethyl]-5-[(5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methyl]-4-hydroxy-3H-pyran-6-one
|
| 别名 |
P 00868554; PF 868554; PF-00868554; PF868554; Filibuvir; 877130-28-4; PF-00868554; (R)-6-Cyclopentyl-6-[2-(2,6-diethylpyridin-4-yl)ethyl]-3-[(5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methyl]-4-hydroxy-5,6-dihydro-2H-pyran-2-one; (R)-6-Cyclopentyl-6-(2-(2,6-diethylpyridin-4-yl)ethyl)-3-((5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methyl)-4-hydroxy-5,6-dihydro-2H-pyran-2-one; 198J479Y2L; (2R)-2-cyclopentyl-2-[2-(2,6-diethylpyridin-4-yl)ethyl]-5-[(5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl)methyl]-4-hydroxy-3H-pyran-6-one; 2H-Pyran-2-one, 6-cyclopentyl-6-(2-(2,6-diethyl-4-pyridinyl)ethyl)-3-((5,7-dimethyl(1,2,4)triazolo(1,5-a)pyrimidin-2-yl)methyl)-5,6-dihydro-4-hydroxy-, (6R)-; PF00868554; PF-868554; Filibuvir
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9855 mL | 9.9275 mL | 19.8551 mL | |
| 5 mM | 0.3971 mL | 1.9855 mL | 3.9710 mL | |
| 10 mM | 0.1986 mL | 0.9928 mL | 1.9855 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00987337 | Completed | Drug: Filibuvir Drug: Placebo |
Hepatitis Hepatitis C |
Pfizer | November 2009 | Phase 2 |
| NCT00823745 | Completed | Drug: [14C]-PF-00868554 | HCV | Pfizer | January 2009 | Phase 1 |
| NCT01210404 | Completed | Drug: filibuvir | Chronic Hepatitis C | Pfizer | February 2011 | Phase 1 |
| NCT01051232 | Completed | Drug: Active Drug: Placebo |
Healthy | Pfizer | February 2010 | Phase 1 |
| NCT00651027 | Completed | Drug: PF-868554 | HEPATITIS C (HCV) | Pfizer | February 2008 | Phase 1 |
|
|
|
|