| 规格 | 价格 | |
|---|---|---|
| 25mg | ||
| Other Sizes |
| 靶点 |
FIPV ( EC50 = 0.78 μM ); RNA-dependent RNA polymerase (RdRp)
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:细胞在所有浓度的 GS-441524 下均出现并正常生长,并且在 24 小时时无法吸收荧光染料 CellTox Green。因此,细胞毒性浓度 50% (CC50) > 100 μM。 GS-441524 的有效浓度 50% (EC50) 经计算为 0.78 μM。细胞测定:为了确定 GS-441524 对 CRFK 细胞的毒性,用 100、33.3、11.1、3.7 或 1.2 μM GS-441524 处理 CRFK 细胞 24 小时。
GC376和GS441524的联合应用增强了Vero E6细胞中抑制严重急性呼吸系统综合征冠状病毒-2的能力[5] 我们评估了GC376、GS441524以及GC376和GS441524(摩尔比:1:1)的联合应用(GC376+GS441524)对Vero E6细胞中活病毒(严重急性呼吸系统综合征冠状病毒-2:HRB26和HRB26M)复制的抑制效果。我们首先在体外测试了这些化合物的细胞毒性。GC376和GS441524在Vero E6细胞中浓度高达250μM时没有产生明显的细胞毒性(CC50>250μM;补充图3)。我们的结果表明,GC376和GS441524对HRB26有效,50%抑制浓度(EC50)值分别为0.643±0.085μM和5.188±2.476μM(图2A、B)。GC376和GS441524对HRB26M也有效,EC50值分别为0.881±0.109μM和5.047±2.116μM(图2D,E)。我们的结果表明,当单独使用这些药物时,GC376抑制病毒(HRB26和HRB26M)复制的能力优于GS441524。我们观察到,GC376+GS441524比单一治疗更有效地抑制HRB26和HRB26M复制,EC50值分别为0.531±0.162μM和0.369±0.068μM(图2C,F)。在冠状病毒的复制过程中,Mpro是第一批被多聚蛋白加工的nsps之一,其他与复制相关的蛋白质,如RdRp,可以在Mpro和PLP蛋白酶的参与下产生[8,9]。这种现象可能是GC376优于GS441524的原因,它们的联合应用可能会产生协同效应,因为这些药物靶向参与病毒复制的不同蛋白质。 GS441524和GC376对猫传染性腹膜炎病毒感染患者的体外抗病毒活性[6] 为了评估口服GC376和GS441524的治疗效果,我们研究了这两种药物在猫肾(CRFK)细胞中的抗病毒活性。使用CCK-8试验测试GC376和GS441524抑制FIPV-rQS79和FIPV-II增殖的能力。我们的实验室之前构建了一种名为FIPV-rQS79的重组病毒,该病毒已被证明在体内可导致100%的死亡率(Delaplace等人,2021;Wang等人,2021)。我们证实了GC376和GS441524对FIPV-rQS79和FIPV-II的抗病毒活性。CRFK细胞通常与药物和病毒一起培养48小时。GC376和GS441524均显示出对FIPV II的作用,EC50值分别为0.9μM和2.142μM(图1A和D)。之前报道的FIPV的EC50值分别为0.78μM(Murphy等人,2018)和0.3μM(Pedersen等人,2018年)。GC376和GS441524也抑制FIPV-rQS79,EC50值分别为1.239μM和2.52μM(图1B和E)。通过CCK-8法测定GC376和GS441524的可能细胞毒性。在CRFK细胞中,在高达100μM的任何浓度下,这两种化合物都没有表现出明显的细胞毒性(图1C和F)。感染后12小时,使用免疫荧光法验证了用GC376和GS441524连续稀释处理的感染细胞中的抗FIPV效力(图1G)。与阳性对照组相比,FIPV-rQS79和FIPV-II感染后,当细胞与1.25μM GS441524或2.5μM GC376(图1G)一起孵育24小时时,没有观察到明显的病理变化。我们的结果表明,GS441524和GC37 |
| 体内研究 (In Vivo) |
10只接受治疗的猫对治疗产生了快速反应,淋巴细胞水平和直肠温度恢复到感染前的水平和两只无症状猫的水平。到目前为止,所有接受过一次或两次治疗的十只猫都保持正常(感染后八个多月)。注射化合物后 10 秒内,某些猫会出现短暂的刺痛反应。局部和短暂的疼痛表现为不寻常的姿势、舔注射部位和/或注射后持续约 30-60 秒的发声。相对于其他动物,某些动物的注射反应更为明显,一次注射与下一次注射的反应不一致,并且随着时间的推移而减少。在 7 天的治疗过程中,NHP 中的 Remdesivir(静脉注射)导致血清中 GS441524 的浓度比 Remdesivir 高 1000 倍。
GC376是一种亚硫酸氢二肽加合物盐,它是3CLpro(3C样蛋白酶)的抑制剂,具有强效的抗病毒和冠状病毒活性,特别是对严重急性呼吸系统综合征冠状病毒。由严重急性呼吸综合征冠状病毒2型(SARS-CoV-2)引起的前所未有的2019冠状病毒病(新冠肺炎)大流行对全球公共卫生构成严重威胁。迫切需要开发针对严重急性呼吸系统综合征冠状病毒2型的有效疗法。在这里,我们使用小鼠适应的严重急性呼吸系统综合征冠状病毒2型感染小鼠模型,评估了瑞德西韦母体核苷酸类似物GS441524和猫冠状病毒前药GC376的抗病毒活性,前者靶向冠状病毒RNA依赖性RNA聚合酶,后者靶向其主要蛋白酶。我们的结果表明,GS441524通过联合鼻内(i.n.)和肌肉内(i.m.)治疗有效地阻断了小鼠上呼吸道和下呼吸道中严重急性呼吸系统综合征冠状病毒2型的增殖。然而,高剂量GC376(i.m.或i.n.和i.m.)的能力弱于GS441524。值得注意的是,低剂量联合应用GS441524和GC376可以通过静脉注射或静脉注射和肌肉注射治疗有效保护小鼠免受严重急性呼吸系统综合征冠状病毒2型感染。此外,我们发现GS441524的药代动力学特性优于GC376,GC376和GS441522的联合应用具有协同作用。我们的研究结果支持在未来的临床研究中进一步评估GC376和GS441524的联合应用[5]。 口服GS441524和GC376在FIPV模型中有效[6] 根据GS441524和GC376 PK分析数据,我们选择了各种口服给药方案。在此阶段,我们评估了不同口服剂量的GS441524(5mg/kg,10mg/kg和20mg/kg)和GC376(15mg/kg,100mg/kg和150mg/kg)在体内对抗FIPV-rQS79。猫被随机分为八组(n=3),口服FIPV-rQS79疫苗。我们以105 TCID50的剂量给猫接种了FIPV-rQS79。 所有接种病毒的猫在GS441524治疗时都出现了发烧和体重急剧下降等症状。接种病毒后,动物体重逐渐下降,治疗后体重逐渐增加,如图3A所示。经过治疗干预后,猫的发烧症状明显减轻,体温逐渐恢复到正常范围,如图3B所示。接种后患者的临床评分显著升高,经GS441524治疗后,临床评分逐渐降低;具体来说,GS441524治疗组的患者症状比阳性对照组的患者轻(图3C)。在观察期间,所有接受GS441524(5mg/kg,与未经治疗的对照组中的感染猫相比,10 mg/kg和20 mg/kg)的存活率显著提高(P<0.05)。20 mg/kg和10 mg/kg的GS441524剂量提供了最大的保护,保护率为100%。5 mg/kg/天的GS441524剂量提供了66%的保护(图3D)。结果表明,口服5mg/kg GS441524是有效的,尽管它不能完全保护FIPV感染的患者。 在FIPV-rQS79感染动物模型中,三种剂量的GS441524治疗30天,150 mg/kg剂量的GC376治疗24小时,可预防FIP相关的死亡率[6] 20只猫暴露于FIPV-rQS79,在出现疾病症状后监测它们对GS441524和GC376治疗的反应(图9)。接种病毒约一周后,大多数猫都出现了临床症状。GS441524和GC376经不同剂量治疗30天后,一些猫恢复健康,但其中4只经口服治疗的猫在治疗后5至6周出现疾病复发,其中2只出现严重的神经症状,表现为瞳孔大小不均和无法控制后腿。除复发性疾病的猫外,其余接受过一次治疗的猫在两个月后保持正常。 与口服或皮下GC376相比,GS441524在药代动力学参数方面表现出更多优势[6] 为了说明口服GC376或GS441524治疗效果的差异,本研究中GS441524的药代动力学参数如表1、表2所示。与GC376相比,GS441524的清除时间更长。当皮下注射后的GC376血浆浓度达到Cmax(18000ηg/mL)时,大约需要8小时才能降至有效浓度以下。但皮下注射后GS441524为2000ηg/mL,给药后约12小时,浓度降至有效浓度以下。尽管GC376的AUC(0-∞)比GS441524高,但其平均停留时间(MRT)也相对较短,表明它比GS441520更容易代谢。 由严重急性呼吸综合征冠状病毒2型(SARS-CoV-2)引起的前所未有的2019冠状病毒病(新冠肺炎)大流行对全球公共卫生构成严重威胁。迫切需要开发针对严重急性呼吸系统综合征冠状病毒2型的有效疗法。在这里,我们使用小鼠适应的严重急性呼吸系统综合征冠状病毒2型感染小鼠模型,评估了瑞德西韦母体核苷酸类似物GS441524和猫冠状病毒前药GC376的抗病毒活性,前者靶向冠状病毒RNA依赖性RNA聚合酶,后者靶向其主要蛋白酶。我们的结果表明,GS441524通过鼻内(i.n.)和肌肉内(i.m.)联合治疗有效地阻断了小鼠上呼吸道和下呼吸道中严重急性呼吸系统综合征冠状病毒2型的增殖。然而,高剂量GC376(i.m.或i.n.和i.m.)的能力弱于GS441524。值得注意的是,低剂量联合应用GS441524和GC376可以通过静脉注射或静脉注射和肌肉注射治疗有效保护小鼠免受严重急性呼吸系统综合征冠状病毒2型感染。此外,我们发现GS441524的药代动力学特性优于GC376,GC376和GS441522的联合应用具有协同作用。我们的研究结果支持在未来的临床研究中进一步评估GC376和GS441524的联合应用[5]。 |
| 酶活实验 |
有几种方法可以测量抑制剂的RdRP酶活性,具体如下:[4]
生化RdRP酶活性测定 (1)RdRP聚合酶延伸模板元件(PETE)测定 由于RdRP在RNA延伸过程中催化NTPs的掺入,因此可以开发一种PETE测定法来检测RdRP的延伸活性。46在这种测定方法中,用荧光探针标记RNA模板5′端的寡核苷酸,用于荧光偏振(FP)测量。荧光探针的极化信号随着RdRP延长新合成的互补RNA链后其迁移率变低而增加。当互补RNA链的延伸停止时,化合物对RdRP活性的抑制降低了FP信号 (2)基于荧光的碱性磷酸酶-聚合酶偶联分析(FAPA) FAPA方法在通过RdRP合成RNA的过程中在底物系统中包括修饰的核苷酸类似物。随着聚合酶反应的进行,修饰核苷酸类似物的掺入导致荧光团的释放,从而允许检测。例如,RdRP催化结合到生长的RNA链中的修饰核苷酸类似物(2-[2-苯并噻唑基]-6-羟基苯并噻唑)缀合的三磷酸腺苷(BBT-ATP),产生BBT的副产物焦磷酸(PPi)。BBTPPi随后与碱性磷酸酶反应以产生高荧光的BBT阴离子 (3)荧光RdRP活性测定 荧光团已被广泛用于RNA和DNA的检测。在该荧光定量RdRP活性测定中,荧光团用于检测来自ssRNA模板的dsRNA形成(图3C)。该测定的一个应用是筛选丙型肝炎病毒(HCV)RdRP的抑制剂。51通过使用聚(C)RNA模板,HCV RdRP催化荧光染料PicoGreen检测到的dsRNA的引物非依赖性合成。51 PicoGreen最初被开发用于量化dsDNA,但随后发现它也优先结合dsRNA而不是ssRNA。51这种测定可以很容易地适用于多种类型病毒的RdRP抑制剂的化合物筛选。除了PicoGreen,其他荧光团也被用于区分dsRNA和ssRNA,它们可用于这种类型的RdRP测定 (4)闪烁邻近度测定(SPA) SPA也已用于HTS的RdRP酶测定。该测定依赖于在3H-GTP存在下使用生物素化引物模板通过RdRP催化将放射性核苷酸掺入新合成的RNA链。链霉亲和素偶联的SPA检测珠在这种放射性酶测定中的应用能够实现均匀的测定检测,避免了原始放射性NTP掺入测定的劳动密集型过滤和洗涤步骤。然而,由于它们是放射性测定,因此需要特定的安全预防措施和废物处理,这可能很不方便,需要加强安全协议。因此,近年来大多数放射性测定已被荧光测定法所取代。 |
| 细胞实验 |
GS-441524在CRFK细胞上用100、33.3、11.1、3.7或1.2μM处理24小时,以评估其毒性[1]。
Vero e6细胞抗病毒活性的评价[5] 按照制造商的说明,使用Cell Titer Glo试剂盒测定细胞存活率。简而言之,将Vero E6细胞接种在具有不透明壁的96孔板中。12-16小时后,加入指定浓度的GC376(0、1、5、10、50、100、500µM)、GS441524(0、1,5、10、50100500µM)和GC376+GS441524(0,0.5、2.5、5、25、50、250µM)24小时。向每个孔中加入Cell Titer-Glo试剂,并使用GloMax 96微孔板光度计测量发光 抗病毒活性实验按照之前的方法进行测定。简而言之,Vero E6细胞用指定浓度的GC376(0,0.5,1,2,4,6,8,10µM)、GS441524(0,0.5,1,2,4,6,8,10µM)和GC376+GS441524(0,0.25,0.5,1,2,3,4,5µM)或单独用载体溶液(12%磺丁基醚-β-环糊精,pH 3.5)预处理1小时。然后用MOI为0.005的HRB26或HRB26M感染细胞,并在37°C下孵育1小时。用PBS洗涤细胞,加入含有指定量的GC376、GS441524和GC376+GS441524的病毒生长培养基或单独的载体溶液。在腹膜后24小时收集上清液,用于在Vero E6细胞中通过PFU测定进行病毒滴定。根据与模拟处理对应物中病毒滴度的比率计算相对病毒滴度。使用GraphPad Prism 7.0对数据进行分析。结果显示为三个独立实验的平均值和标准偏差 在96孔透明平底板中,接种Vero E6细胞(3000个细胞/孔),在37°C下用5%的CO2孵育24小时。孵育后,使用0.1的多重感染(MOI)感染细胞。允许严重急性呼吸系统综合征冠状病毒2型在37°C下吸附1小时。在去除病毒接种物后,用含有三倍稀释的莫奈韦(0.62-50μM)、奈马替韦(0.62-50μM)和GC376(0.21-16.7μM)的培养基覆盖细胞。每个平板都含有模拟感染细胞、感染阳性对照(仅限严重急性呼吸系统综合征冠状病毒2型)和阴性对照(仅含化合物)。在37°C和5%CO2下孵育48和72小时后,使用MTT还原法评估细胞的存活率。 GS441524和GC376在CRFK细胞中的细胞毒性[6] 将CRFK细胞接种到96孔板中,并在含有10%胎牛血清(FBS)的DMEM(Gibco,USA)中生长。当细胞形成单层时,用2%FBS和不同浓度的GC376(0.3125µM、0.625µM、1.25µM和2.5µM)或GS441524(0.3125μM、0.625M、1.25μM和2.5μM)代替培养基。使用含有0.4%DMSO的DMEM作为空白对照。在含有5%CO2的气氛下,将细胞在37°C下孵育48小时,然后用磷酸盐缓冲盐水(PBS)洗涤两次。然后加入不含FBS的MEM(100µL)和CCK-8(10µL),将细胞在37°C下孵育1-4小时。使用FLUOstar Omega读取450 nm处的光密度(OD)。使用以下方程式计算细胞存活率: 细胞存活率=[OD(化合物)-OD(空白)]/[OD(对照)-OD-(空白)]×100%· EC50值是使用GraphPad Prism软件版本8.0.2计算的。 评估GC376和GS441524对FIPV-rQS79和FIPV-II的体外抗病毒作用(0.01 MOI);将不同浓度的GC376或GS441524加入含有CRFK细胞单层的96孔板中,在37°C和5%CO2的气氛下孵育细胞28小时。在五个重复孔中测试每种药物浓度,并使用0.4%DMSO作为空白对照。EC50值通过CCK-8测定法测定。 间接免疫荧光法(IFA)[6] 通过将细胞接种在玻璃盖玻片上并允许其生长直至达到50%的膜融合,对FIPV感染期间以及经GS441524和GC376处理的CRFK细胞中FIPV N蛋白表达进行免疫荧光分析。简而言之,将盖玻片上的CRFK细胞单层在37°C下接种FIPV II或FIPV-rQS79(MOI=0.01)24小时。用PBS洗涤后,我们将CRFK细胞在4%多聚甲醛中固定20分钟,然后在室温下在0.3%Triton-X-100中透化30分钟,并在37°C下用5%BSA封闭30分钟。用PBS洗涤后,我们在4°C下用抗FIPV N单克隆抗体孵育细胞过夜。用PBS吐温-20(PBST)洗涤3次后,我们将细胞与山羊抗兔488(1:1000)在37°C下孵育1.5小时。我们在室温下用DAPI(1:1000)再次孵育15分钟。然后用PBST洗涤三重染色的细胞三次,我们在高倍镜下捕获图像进行分析。 |
| 动物实验 |
Cats: Three days after unambiguous clinical evidence of FIP (days 12-19 post infection), the 10 cats that showed disease signs were split into two groups and treated with either 5 mg/kg (Group A; n=5) or 2 mg/kg (Group B; n=5) GS-441524 SC q24 h. The two cats that do not show any symptoms of the disease act as controls for normal rectal temperature and blood lymphocyte counts[1].
In vivo toxicity study of GC376 and GS441524[5] The toxicity studies were performed in 4- to 6-week-old female BALB/c mice. BALB/c mice were assigned to four groups (five mice per group), one mock group (i.m. administration of solvent) and three i.m. administered groups: GC376 (40 mM/l, 100 µl), GS441524 (40 mM/l, 100 µl) and GC376 + GS441524 (20 mM/l, 100 µl), respectively. Mice in the mock and experimental groups were weighed daily for 15 days. In addition, blood samples were collected at 0, 5, 10 and 15 days after administration. Various blood chemistry values or blood cell counts were performed at Wuhan Servicebio Biological Technology Co., Ltd. The data were analyzed using GraphPad Prism 7.0. In vivo antiviral study of GC376 and GS441524[5] Firstly, groups of six 4- to 6-week-old female BALB/c mice were treated i.m. with a loading dose of GC376 (40 or 8 mM/l, 100 µl), GS441524 (40 or 8 mM/l, 100 µl) and GC376 + GS441524 (20 or 4 mM/l, 100 µl), followed by a daily maintenance dose. Alternatively, mice were treated intranasally with a single treatment (GC376, 20 mM/l, 50 µl; GS441524, 20 mM/l, 50 µl; GC376 + GS441524, 10 mM/l, 50 µl) or a combination of GC376 (20 mM/l, 50 µl, i.n. and 40 mM/l, 100 µl, i.m.), GS441524 (20 mM/l, 50 µl, i.n. and 40 mM/l, 100 µl, i.m.) and GC376 + GS441524 (10 mM/l, 50 µl, i.n. and 20 mM/l, 100 µl, i.m.), followed by a daily maintenance dose. As a control, mice were administered vehicle solution (12% sulfobutylether-β-cyclodextrin, pH 3.5) daily. One hour after administration of the loading dose of GC376, GS441524 and GC376 + GS441524 or vehicle solution, each mouse was inoculated intranasally with103.6 PFU of HRB26M in 50 μl. Three mice from each group were euthanized on days 3 and 5 p.i. The nasal turbinates and lungs were collected for viral detection by qPCR and PFU assay according to previously described methods]. The amount of vRNA for the target SARS-CoV-2 N gene was normalized to the standard curve from a plasmid containing the full-length cDNA of the SARS-CoV-2 N gene. The assay sensitivity was 1000 copies/ml. The data were analyzed using Microsoft Excel 2016 and GraphPad Prism 7.0. Pharmacokinetics study of GC376 and GS441524 in BALB/c mice and SD rats[5] Healthy SPF BALB/c mice (7-8 weeks) and SD rats (4-6 weeks) were used in a single-dose PK study. At time point zero, the BALB/c mice and SD rats of groups A, B and C (each group including twenty BABL/c mice or five SD rats) received i.m. injections of GC376 (111 mg/kg), GS441524 (67 mg/kg) and GC376 + GS441524 (55.5 + 33.5 mg/kg), which are the same doses according to in vivo antiviral study. The blood was collected at 0, 0.083, 0.25, 0.5, 1, 2, 4, 8, 12 and 24 h and placed in a precooled polypropylene centrifuge tube containing 3.0 µl of 40% EDTAK2. Then, the whole blood was centrifuged at 7800 g/min for 10 min at 4°C. Plasma was collected and stored in a freezer at −80°C. Plasma drug concentration was analyzed using LC-MS/MS. Pharmacokinetic parameters were calculated using WinNonlin software (version 6.4), and a non-atrioventricular model was used for data fitting. The data were analyzed using Microsoft Excel 2016 and GraphPad Prism 7.0. Pharmacokinetic studies of GC376 and GS441524 in cats [6] A pharmacokinetic (PK) study was performed in laboratory cats to determine the efficacy of oral GS441524 and GC376. GS441524 was dissolved at a concentration of 12 mg/mL in 5% ethanol, 30% propylene glycol, 45% PEG 40%, and 20% water and adjusted to pH 1.9 with concentrated HCl. All animals were randomly divided into the following three groups: A (n ≧ 3; IV administration), B (n ≧ 3; SC administration), and C (n ≧ 3; PO oral administration). At time point zero, Group A cats were administered 5 mg compound/kg body weight intravenously, while Group B cats received 5 mg compound/kg subcutaneously. Serial 0.5 mL whole blood samples in EDTA were obtained from the radial vein of the forelimb from each cat at 0.25, 0.5, 1, 2, 4, 6, 8, 12 and 24 h. After collection, blood samples were immediately placed on ice and centrifuged at 5000 rpm for 5 min. The isolated plasma was pipetted into a 1.5 mL microcentrifuge tube and frozen at − 80 °C for further analysis of free GS441524. All samples were assessed using LCsingle bondMS-MS to detect the concentrations. Healthy cats (1–3 years old, 2.0–4.5 kg) were randomly assigned to eight groups (four animals per group) once they had been confirmed to be virus-free by a neutralizing antibody test. Meanwhile, we also measured their liver and kidney functions, and the test results were normal, ensuring that the test cats had the ability to absorb and metabolize drugs normally. The effect of GC376 or GS441524 oral administration was investigated first in FIP. After viral infection, animals with FIPV-rQS79 in the treatment groups received oral doses of GS441524 (20 mg/kg, 10 mg/kg or 5 mg/kg) or GC376 (150 mg/kg, 100 mg/kg or 15 mg/kg) in PBS (500 µL). The control group received the same volume of PBS. On day 0, the cats were infected by oral administration of FIPV-rQS79 (105 × TCID50) in DMEM (1000 µL). The therapeutic effect of GC376 and GS441524 after the onset of infection was examined next. Clinical signs and survival rates of the animals were monitored as described previously, and cat health was assessed every day [6]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
GS-441524 has been found to transport poorly into cells compared to remdesivir. Metabolism / Metabolites GS-441524 is phosphorylated 3 times to form the active nucleoside triphosphate. Pharmacokinetics of GS441524 and GC376 by different routes of administration and with different dosages [6] To determine the oral dose of GC376 and GS441524, we tested the pharmacokinetics of the two drugs given by different administration routes (including subcutaneous, oral and intravenous injection) in healthy adult cats. The concentration-time curves of GS441524 and GC376 after each different administration route are shown in Fig. 2 A and C. The pharmacokinetic samples were assessed using LC-MS-MS to detect the concentrations. According to the results, oral GS441524 has the same area under the curve as that given subcutaneously, indicating that changing the method of administration did not affect drug absorption; thus, GS441524 can be administered orally at the doses reported in the literature. In contrast, the absorption of oral GC376 was significantly lower than when given by subcutaneous administration; thus, oral GC376 may require higher doses. Due to the low solubility of GS441524, we hypothesized that the reduced drug solubility would affect drug absorption. To test this hypothesis, we evaluated the pharmacokinetic differences between oral GS441524 and GC376 given as a powder or in solution. The results showed that compared with liquid GS441524, GS441524 powder had significantly lower drug absorption; however, there was no significant difference in drug AUC when comparing oral and subcutaneous administration of GS441524 (Fig. 2 G and H). Conversely, compared with liquid GC376, there was no change in the drug absorption of GC376 powder; however, there was a significant difference in drug absorption when oral and subcutaneous administrations of GC376 were compared (Fig. 2E and F). In this study, the solution was used as the primary dosage form for subsequent animal tests. Pharmacokinetics study of GC376 and GS441524 alone or in combination [5] To further examine the potential of GC376 and GS441524, we evaluated their pharmacokinetic (PK) properties in SPF BALB/c mice and SD rats following i.m. administration of GC376 (111 mg/kg), GS441524 (67 mg/kg) and GC376 + GS441524 (55.5 + 33.5 mg/kg), which are the same doses according to in vivo antiviral study. In mice, the PK results showed that GC376 and GS441524 were rapidly absorbed after i.m. administration, and the peak plasma level was reached 0.22 ± 0.07 h and 0.80 ± 0.24 h after injection, respectively (Figure 5A, B and Table 1). Because the i.m. administered dose of GC376 was approximately 1.7-fold that of GS441524, we found that the maximum detected plasma drug concentration (Cmax) of GC376 (46.70 ± 10.69 μg/ml) was approximately 1.2-fold that of GS441524 (39.64 ± 2.93 μg/ml) (Table 1). However, the value of the area under the curve (AUC0−t) of GS441524 (AUC0−t = 106.82 ± 16.79) was approximately 1.9-fold that of GC376 (AUC0−t = 55.29 ± 11.26). Meanwhile, we observed that the clearance rate of GC376 (CL/F, 1985 ± 485 ml/h/kg) in plasma was approximately 3.1-fold that of GS441524 (CL/F, 639 ± 119 ml/h/kg) (Table 1). Besides, the PK results in SD rats showed that the Tmax of GC376 and GS441524 were 1.30 ± 0.60 h and 2.00 ± 1.10 h, respectively (Figure 5D, E and Table 2). Compared with mice, the utilization efficiency of GS441524 in vivo is significantly higher than that of GC376 in SD rats. We found that the maximum detected plasma drug concentration (Cmax) of GC376 (12.56 ± 1.90 μg/ml) was approximately 2.5-fold lower than GS441524 (30.96 ± 8.40 μg/ml) (Table 2). The value of the area under the curve (AUC0−t) of GS441524 (AUC0−t = 183.33 ± 64.36) was approximately 2.0-fold higher than GC376 (AUC0−t = 92.14 ± 9.99). We also observed that the clearance rate of GC376 (CL/F, 1208 ± 122 ml/h/kg) in plasma was approximately 2.9-fold that of GS441524 (CL/F, 423 ± 186 ml/h/kg). |
| 毒性/毒理 (Toxicokinetics/TK) |
GC376 and GS441524 did not produce obvious cytotoxicity at concentrations up to 250 μM in Vero E6 cells (CC50 > 250 μM; Supplementary Figure 3). [5]
The possible cytotoxicity of GC376 and GS441524 were determined by CCK-8 assay. Neither of the compounds showed obvious cytotoxicity at any of the concentrations up to 100 μM in CRFK cells (Fig. 1 C and F).[6] |
| 参考文献 | |
| 其他信息 |
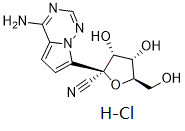
GS-441524 is a C-nucleoside analog that is (2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile substituted by a 4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl group at position 2. It is the active metabolite of remdesivir and exhibits a broad range of inhibitory activity against various RNA viruses including HCV, parainfluenza and SARS-CoV. It has a role as a drug metabolite, an antiviral agent and an anticoronaviral agent. It is a pyrrolotriazine, a nitrile, a C-nucleoside and an aromatic amine.
GS-441524 is an adenosine nucleotide analog antiviral, similar to [remdesivir]. This molecule was patented in 2009. In vitro studies of GS-441524 have determined it has a higher EC50 than remdesivir against a number of viruses, meaning GS-441524 is less potent. GS-441524 continues to be studied in the treatment of Feline Infectious Peritonitis Virus, a coronavirus that only infects cats. Mechanism of Action GS-441524 is phosphorylated 3 times to form the active nucleoside triphosphate, which is incorporated into the genome of virions, terminating its replication. These results indicated that the utilization efficiency of GS441524 in vivo is significantly higher than that of GC376. This finding may be one of the reasons for the poor ability of GC376 to inhibit SARS-CV-2 in vivo. The previous results showed that GC376 targeting the FIPV 3CLpro could effectively reduce the virus load in the macrophages from the ascites of cats with the duration of antiviral treatment [17,19]. Therefore, we speculate that GC376 can effectively inhibit the proliferation of coronaviruses (FIPV and SARS-CoV-2), but cannot quickly clear the virus from infected tissues. During continuous administration, GC376 needs to maintain an effective concentration for a long time to inhibit virus proliferation in the infected tissue. However, compared with GS441524, GC376 could be quickly cleared in the BALB/c mice and SD rats. Besides, the nasal turbinate and lung are the main target organs for SARS-CoV-2 proliferation, there is a large amount of SARS-CoV-2 in these tissues. Therefore, it is difficult for GC376 to completely inhibit the proliferation of SARS-CoV-2 in the mice nasal turbinates and lungs. Furthermore, we found that the combined application of GC376 and GS441524 extended T1/2 from 1.51 ± 0.16 h to 1.67 ± 0.24 h and the residence time of GS441524 (MRT0−t from 2.07 ± 0.42 h to 2.37 ± 0.73 h) in SPF BALB/c mice (Figure 5C and Table 1). Similarly, the PK results showed that the combined application of GC376 and GS441524 extended T1/2 from 3.80 ± 1.17 h to 5.13 ± 2.56 h and the residence time of GS441524 (MRT0−t from 4.50 ± 1.11 h to 6.03 ± 1.37 h) in SPF SD rats (Figure 5F and Table 2). Moreover, the PK study results showed that GC376 reached Cmax earlier (Tmax = 0.25 h in mice and T max = 1.40 ± 0.49 h in SD rats) than GS441524 (Tmax = 0.55 ± 0.24 h in mice and T max = 3.40 ± 1.20 h in SD rats) to produce a synergistic effect (Figure 5C, F). When these agents were combined, GC376 was the first drug to inhibit SARS-CoV-2 replication. After the plasma concentration of GC376 decreased, GS441524 reached its Cmax (Figure 5C, F and Tables 1 and 2) to produce a continuous inhibition of SARS-CoV-2 proliferation and maintain the effective concentration for a longer time. This phenomenon may explain why the combined application of GC376 and GS441524 was better than single application alone. In summary, we assessed the efficacy of GC376 and GS441524 to inhibit SARS-CoV-2 replication using a mouse-adapted virus infection model. Importantly, we found that intranasal administration of GS441524 and GC376 + GS441524 significantly prevents the replication of virus in the upper respiratory tract, and the efficacy of GC376 + GS441524 to inhibit the viral replication in the lower respiratory tract significantly better than that of GS441524. Combined i.n. and i.m. administration of GS441524 and GC376 + GS441524 effectively protected mice against HRB26M infection in the upper and lower respiratory tracts, but GC376 alone failed to block the proliferation of SARS-CoV-2 in mice. Compared with GC376 and GS441524 alone, the dosage of GC376 + GS441524 is halved, these results showed an additive effect of the combined application of the Mpro and RdRp inhibitors, so it should be developed and considered for future clinic practice. [5] FIP caused by FIPV threatens feline health. GS441524 and GC376 have effective for FIPV by inhibiting virus replication, subcutaneous injection as administration way has limitations, oral administration has its advantages, including patient compliance, convenience, cost, and ease of storage (Shriya S. Srinivasan, 2022). Oral administration is expected to be a new method for FIP treatment. Thus, our study demonstrates the efficacy of oral GS441524 and GC376 against lethal recombinant FIPV-rQS79 in vivo and in vitro. First, we demonstrated that these two drugs can effectively inhibit two kinds of FIPV viruses in cell culture. The two drugs both had broad-spectrum antiviral effects against type FIPV-rQS79 and type FIPV II in vitro. Pharmacokinetics (PK) is closely related to pharmacodynamics. Testing pharmacokinetics can determine drug processes relevant to cats (absorption, distribution, metabolism and excretion) (Asif et al., 2005). Through a pharmacokinetic study, we found that compared with GS441524, GC376 was metabolized faster. Compared with GS441524, GC376 also had a faster plasma elimination half-life and a shorter MRT. Compared with subcutaneous injection, oral administration of GC376 significantly increased the overall clearance rate and apparent distribution volume (P < 0.0001). GC376 is a covalent peptidomimetic inhibitor, and it may be modified from a peptide inhibitor, so there are more unstable chemical bonds. Previous studies have also shown that the bisulfite adducts readily revert to the aldehyde forms in water, which are readily epimerized and form the active inhibitory stereoisomer (Vuong et al., 2021). The above two reasons suggest that the use of GC376 may lead to unsatisfactory clinical results. However, when using GS441524, there were no significant differences in these PK parameters when comparing the subcutaneous and oral routes. GS441524 exhibited favorable PK parameters, and GS441524 given by either subcutaneous injection or oral administration led to the same area under the curve (same bioavailability). This study is different from previous reports in humans and mice because oral bioavailability varies greatly in different species, with F values of 33% in rats, 85% in dogs, and 8.3% in cynomolgus monkeys (Davis et al., 2021; Humeniuk et al., 2020; Li et al., 2022; Wei et al., 2021; Xie and Wang, 2021). The results indicated that metabolic differences are one of the reasons for the differences in the effects of these drugs in vivo. These results preliminarily explain why GS441524 has better efficacy than GC376 in vivo. Solubility is one of the factors affecting drug absorption, because of the low solubility of GS441524, we suspect that preparation factors also play a role in oral absorption. The results show that compared with liquid GS441524 given orally, GS441524 powder given orally had significantly lower drug absorption; however, there was no significant difference in drug absorption when comparing oral and subcutaneous administrations of GS441525. Conversely, compared with liquid GC376 given orally, GC376 powder given orally did not alter drug absorption. Therefore, solubility affects GS441524 absorption but not GC376 absorption. In vivo study, we found that oral GS441524 has efficacy regardless of the dose, but oral GC376 only has efficacy at the high dose (150 mg/kg). Although the two drugs have good inhibitory effects in vitro, the effects of the two drugs are significantly different in vivo. Drugs can be introduced into the body through different routes, including enteral, parenteral, and topical routes. Each of the different routes of administration has a specific purpose, advantages and disadvantages. Fundamentally, the accessibility of the respective target site of the drugs and the effectiveness of drug treatment are both strongly dependent on the route of administration. Among the various routes of administration, oral dosing has attracted the most attention because of its advantages, including patient compliance, convenience, cost, and ease of storage, transport and administration (Mignani et al., 2013). Although oral administration is the optimal method for small molecules, there are some limitations to its application. Compared with other routes, the mechanism of drug absorption following oral administration is more complex and influenced by many factors (for example gastrointestinal motility, gastric emptying rate and presence of food). Orally administered drugs must overcome the harsh acidic environment of the stomach and be able to be dissolved in GI fluid and remain stable among dynamic intestinal microbiota; additionally, these drugs must evade degradative enzymes that can penetrate the viscous mucus barrier and efflux pumps to achieve therapeutic bioavailability (Srinivasan et al., 2022). Overcoming these barriers is difficult. Apart from the oral route, the drug can be injected subcutaneously through the small blood vessels under the skin and into the circulatory system to exert its effect; thus, this method of administration relatively quickly achieves efficacy, but it also has a stimulating effect, and the route is painful for animals. Additionally, the production costs and quality requirements for injection solutions are high. These pathway differences lead to differences in the absorption and metabolism of drugs. Therefore, we should choose a better route of drug administration based on various factors. At the same time, pharmacokinetics provides guidance for clinical drug use. The pharmacokinetic data can also be used to provide ideas for drug structure optimization. We can further reduce the existing disadvantages of the compound through structural modification and hopefully develop more advantageous anti-FIPV compounds. For example, the structure of GC376 was optimized by Liu et al., who found that additional modification of the benzyl group may lead to a stronger bond or even additional hydrogen bonds with Mpro. Compound NK-0163 has the advantage of a long half-life in critical tissues such as the lung, although this halogen substitution may have altered its pharmacokinetics (Liu et al, 2022). Quan et al. reported that a series of potent α-ketoamide-containing compounds, specifically Y180, had superior bioavailability in rodents and nonrodents (Quan et al., 2022). Previous studies also have reported that GS441524 has good antiviral activity and has the potential to be given by oral administration. However, the unfavorable oral PK prevented its further development into an oral drug (Li et al., 2022). To address this issue, Wei et al. reported a series of GS441524 analogs with modifications on the base or the sugar moiety, as well as some prodrug forms, in which 3′-isobutyryl ester 5a, 5′-isobutyryl ester 5c, and isobutyryl ester 5 g hydrobromides have better oral bioavailability than GS441524 (F=71.6%, 86.6% and 98.7%, respectively)(Wei et al., 2021). The above modifications of GC376 and GS441524 can be further tested, it may be improved the therapeutic effect of GC376 and GS441524 drugs on patients with FIPV. In conclusion, this study is the first to report that oral GS441524 and GC376 can effectively treat FIPV infection in an animal model. Our research demonstrated that oral dosing can be used to replace subcutaneous injections, although we still need to solve the problems that exist with new approaches or new methods. Overall, GS441524 and GC376 completely inhibited FIPV-rQS79 and FIPV II replication in CRFK cells. Our study also verified the effect of oral GC376 and GS441524 treatment and confirmed the oral availability of GS441524 and GC376. Through PK studies, we determined the absorption, distribution, metabolism and excretion of the two drugs in cats. Additionally, the PK results further explained the reason for the differences in efficacy between the two drugs in vivo and provided insights into and directions for drug optimization and transformation.[6] |
| 分子式 |
C12H14CLN5O4
|
|---|---|
| 分子量 |
327.723660945892
|
| 元素分析 |
C, 43.98; H, 4.31; Cl, 10.82; N, 21.37; O, 19.53
|
| CAS号 |
2378280-82-9
|
| 相关CAS号 |
1191237-69-0;2378280-82-9 (HCl);2378280-83-0 (sulfate);
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| InChi Key |
RUKJXQWRCFJDMA-BITRNDABSA-N
|
| InChi Code |
InChI=1S/C12H13N5O4.ClH/c13-4-12(10(20)9(19)7(3-18)21-12)8-2-1-6-11(14)15-5-16-17(6)8/h1-2,5,7,9-10,18-20H,3H2,(H2,14,15,16)1H/t7-,9-,10-,12+/m1./s1
|
| 化学名 |
(2R,3R,4S,5R)-2-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-carbonitrile
hydrochloride
|
| 别名 |
GS-441524 HCl; GS-441524 hydrochloride; GS-441524; GS441524;
GS 441524; Remdesivir-metabolite, GS-5734-metabolite;
GS5734-metabolite; GS 5734-metabolite
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0514 mL | 15.2569 mL | 30.5139 mL | |
| 5 mM | 0.6103 mL | 3.0514 mL | 6.1028 mL | |
| 10 mM | 0.3051 mL | 1.5257 mL | 3.0514 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04859244 | Completed | Drug: GS-441524 | COVID-19 | Copycat Sciences LLC | January 1, 2021 | Phase 1 |