| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Laminin I
|
|---|---|
| 体外研究 (In Vitro) |
通过与YIGSR(150μg/ml)一起孵育,层粘连蛋白I的细胞附着减少到对照的约50%(见图6A),而对照肽YIGSK没有影响
与YIGSR一起孵育后,他的附着减少不是肽本身的中毒作用,因为补充和不补充肽YIGSR的内皮细胞的增殖试验显示了相同的生长模式(数据未显示) 在接种后16小时加入高浓度肽(150μg/ml)并随后施加剪切应力(16dyn/cm2),也得到了类似的结果。经过6小时的剪切应力后,在存在YIGSR的情况下,80%的细胞被移位,而没有肽的情况下只有不到10%的细胞丢失(图6B) 因此,我们在以下Northern实验中使用了仅35μg/ml的抑制肽YIGSR。经过3天的细胞培养,通常需要达到融合细胞层的时间,内皮细胞已经合成了额外的基质蛋白(见图1A),可以承受剪切应力,施加的机械力冲走了不到10%的细胞群(数据未显示) 为了证明肽YIGSR对内皮细胞没有额外影响,进行了增殖实验。用3ng/ml碱性成纤维细胞生长因子刺激在含1%血清的培养基中饥饿16小时的内皮细胞。在有或没有肽YIGSR的情况下孵育的细胞之间没有差异,表明YIGSR不会改变存活率和增殖(数据未显示)。[2] 抑制剪切应力诱导的eNOS表达[2] 半定量逆转录酶PCR和Northern分析均表明,在与肽YIGSR(n=5)孵育期间,剪切应力后eNOS表达的原始2倍层粘连蛋白依赖性增加被消除(见图7)。相比之下,肽YIGSK的对照实验对剪切应力依赖性表达模式没有显著影响(2.3倍诱导,n=6)。 剪切应力后用YIGSR孵育对eNOS蛋白的影响[2] 为了验证之前的mRNA分析结果,我们还对eNOS蛋白进行了Western印迹。不同涂层I型胶原、纤维连接蛋白和单独玻璃的蛋白质印迹实验表明,在6小时的剪切应力后,eNOS蛋白没有被诱导(图3B显示了n=2的代表性条带)。然而,如图3A所示,在层粘连蛋白I上生长的内皮细胞显示出eNOS的显著增加(n=4)。在剪切应力期间(n=3),用LBP抑制肽YIGSR孵育细胞完全消除了这种蛋白质诱导(见图8、A和B),而用对照肽YIGSK孵育则没有效果(n=3)。 |
| 体内研究 (In Vivo) |
多聚体YIGSR(Ac-Y16)肽对SCID小鼠肿瘤生长和白血病细胞扩散的影响。在SCID小鼠中皮下注射人B前白血病NALM6细胞(2×106)和Matrigel(2mg),有或没有多聚体YIGSR肽(Ac-Y6)。作为对照,Ac-S16还注射了Matrigel和NALM6细胞。9周后,分析原发性肿瘤的生长和白血病细胞向脾、肝、肺、肾、脑和骨髓的浸润。有或没有Ac-Y16或Ac-S16的小鼠皮下肿瘤的重量存在显著差异(P=0.0004)(图2)。当同时注射1.5或2.0mg Ac-Y16时,在小鼠中没有观察到肿瘤形成。1毫克Ac-Y16显著抑制肿瘤生长(P<0.01)90%以上。相比之下,当1.5mg Ac-S16与白血病细胞共注射时,观察到对肿瘤生长的抑制作用较小。然而,这些差异并不显著(图2)。为了检查Ac-Y16对肿瘤生长的抑制是否是由于抑制细胞增殖、凋亡导致的细胞死亡或两者兼而有之,我们测量了白血病细胞的有丝分裂指数和皮下肿瘤的凋亡细胞数量。在有和没有Ac-Y16或Ac-S16的皮下肿瘤中,有丝分裂指数和凋亡细胞数量没有显著差异(数据未显示)。这些发现通过MIB1单克隆抗体染色检测有丝分裂细胞得到了证实。通过流式细胞术分析评估白血病细胞在外周器官中的浸润情况。图3给出了从未经治疗的对照小鼠和含有白血病细胞和Matrigel的小鼠中获得的代表性样本的流式细胞术图谱。未经治疗的小鼠的所有器官对CD10和CD19抗体都没有反应或反应性很低,而在含有白血病细胞和Matrigel的小鼠器官中检测到大量的CD10+CD19+细胞。图4显示了六组不同小鼠所有器官中白血病细胞的浸润百分比。单独使用Matrigel的所有小鼠的脾、肝、肺、肾、脑和骨髓中均观察到高水平的白血病浸润。有和没有Ac-Y16或Ac-S16的小鼠在所有器官中的白血病细胞浸润存在显著差异:脾脏P=0.0075,肝脏P=0.0001,肺P=0.0001,肾脏P=0.0018,脑P<0.0001,骨髓P<0.0001。两毫克Ac-Y16完全抑制了所有器官中白血病细胞的浸润。在1.5 mg Ac-Y16时,脾脏(P<0.05)和其他器官(P<0.01)的白血病浸润受到显著抑制,如图3所示。在所有检查的器官中,大脑对测试肽的存在最敏感。用0.5mg或1.0mg的Ac-Y16治疗的小鼠脑中仅观察到白血病细胞的低浸润。相比之下,用1.5mg的Ac-S16治疗,仅在BM中观察到白血病浸润的显著抑制(P<0.01)。白血病细胞的扩散也通过脾脏、脑和骨髓中人类β-肌动蛋白mRNA表达的RT-PCR分析进行了评估(图5)。在仅用Matrigel治疗的小鼠脾脏、脑和骨髓中检测到人β-actin mRNA。在用1.0 mg Ac-Y16治疗的小鼠中,仅在脾脏和骨髓中观察到人β-actin mRNA的表达;用1.5mg Ac-Y16处理的小鼠在所有器官中都检测不到β-actin mRNA。相比之下,在1.5 mg Ac-S16的小鼠中检测到人β-肌动蛋白mRNA。用Ac-S16处理的小鼠BM,通过流式细胞术仅显示少量白血病细胞浸润,通过RT-PCR也表达了人β-肌动蛋白。因此,Ac-Y16以剂量依赖的方式抑制了肿瘤生长和白血病细胞向外周器官的扩散[1]。
|
| 细胞实验 |
Attachment Experiments [2]
根据Iwamoto等人的论文进行了附着实验。在播种时,加入150μg/ml的YIGSR肽或YIGSK作为对照。在不同间隔后,通过在10个随机分布的光场中计数细胞,在显微镜下记录细胞附着情况 在存在剪切应力的情况下,对YIGSR对附着的急性影响进行了如下测试。使内皮细胞与层粘连蛋白I粘附16小时,在剪切应力之前加入肽YIGSR(150μg/ml)。所有附着实验均一式三份 为了测试通过67 kDa LBP对剪切应力介导的信号转导的影响,在初始接种后3天和剪切应力开始前不久,以较低的浓度(各35μg/ml)加入针对LBP的抑制剂肽YIGSR和对照肽YIGSK。 |
| 动物实验 |
Engraftment of pre-B leukaemic cells in SCID mice [1]
NALM6, a human pre-B ALL cell line (Uckun et al, 1992), was maintained in RPMI-1640 medium with 10% FBS. The SCID mice were bred and maintained in defined flora colonies. The engraftment of NALM6 cells in SCID mice was performed as described previously (Ishii et al, 1995). Briefly, NALM6 cells (2 × 106 ) and 2 mg of Matrigel were co-injected subcutaneously (s.c.) with 0.5–2.0 mg of Ac-Y16 or 1.5 mg of Ac-S16 in 8–10-week-old SCID mice. After 9 weeks, the mice were sacrificed and the tumour weights in mice with or without either Ac-Y16 or As-S16 were measured. The s.c. tumour, spleen, liver, lung, kidney, brain and bone marrow (BM) in each mouse were excised and minced with a 40-mesh screen. The cell suspensions of each organ were washed several times with medium and then subjected to flow cytometry. The number of mice analysed in the study was 5–8 in each group. Total RNA was extracted from these tissues for analysis by the reverse transcriptase polymerase chain reaction (RT-PCR) method |
| 参考文献 |
[1]. The laminin-derived peptide YIGSR (Tyr–Ile–Gly–Ser–Arg) inhibits human pre-B leukaemic cell growth and dissemination to organs in SCID mice. Br J Cancer. 1999 Aug; 80(12): 1898–1904.
[2]. The 67-kDa laminin-binding protein is involved in shear stress-dependent endothelial nitric-oxide synthase expression. J Biol Chem. 1999 Jun 4;274(23):15996-6002. |
| 其他信息 |
The YIGSR (Tyr–Ile–Gly–Ser–Arg) laminin β1 chain sequence has an inhibitory effect on tumour growth and the metastasis of melanoma and fibrosarcoma cells. In the present study, we investigated whether the multimeric YIGSR peptide (Ac-Y16) has an antiproliferative effect and/or prevents the metastasis of human pre-B acute lymphoblastic leukaemia cells (NALM6) in severe combined immune deficient (SCID) mice. In in vitro studies, Ac-Y16 significantly inhibited leukaemic cell colony formation and the invasion of NALM6 cells in a Matrigel-based assay. The tumour growth and leukaemic infiltration in peripheral tissues were also analysed in SCID mice 9 weeks after NALM6, Matrigel and Ac-Y16 were subcutaneously co-injected. The weight of the subcutaneous tumours was significantly suppressed by Ac-Y16 in a dose-dependent manner. Flow cytometry analysis showed that the leukaemic infiltration was significantly inhibited in all organs with 1.5–2.0 mg of Ac-Y16. Leukaemic infiltrations in the brain were inhibited with 0.5 mg of Ac-Y16, and those in brain and bone marrow were also inhibited with 1.0 mg of Ac-Y16. With Ac-S16, a control-scrambled peptide, the only significant inhibition of the leukaemic infiltration was observed in bone marrow at a much higher dose. These data suggest that the multimeric YIGSR peptide can inhibit the tumour growth and metastasis of leukaemic cells and may be useful as a potential therapeutic reagent for leukaemic infiltrations.[1]
The Tyr–Ile–Gly–Ser–Arg (YIGSR) sequence derived from the laminin β1 chain has been shown to inhibit tumour growth and metastasis (Graf et al, 1987; Iwamoto et al, 1987; Saiki et al, 1989; Fridman et al, 1990). It was reported that the multimeric YIGSR polypeptide greatly enhanced the inhibition of tumour growth and metastasis (Nomizu et al, 1993). Although the mechanisms of this effect of YIGSR are still not clear, recent results have suggested that apoptosis may play a role in the anti-metastatic and antitumour effects associated with multimeric YIGSR peptide in HT1080 human fibrosarcoma cells (Kim et al, 1994). However, cell type-specific apoptosis by YIGSR has not been demonstrated (Kim et al, 1994). It was also reported that YIGSR reduces angiogenesis (Sakamoto et al, 1991; Iwamoto et al, 1996). The interaction between Matrigel and leukaemic cells can also facilitate a proliferative response (Sterling-Levis et al, 1993; Ishii et al, 1995; Yan et al, 1996). As shown in a previous report (Blase et al, 1996), NALM6 expressed high levels of VLA-α3, -α4, -α5 and -α6, and VLA-β1. VLA-α6, which is usually expressed on pre-B leukaemic cells, interacts with laminin (Hynes, 1992). In fact, NALM6 cells mainly adhere to laminin, and this binding is significantly reduced by the β1 and α6 monoclonal antibodies (Blase et al, 1996). We also found that Ac-Y16 has similar activity for NALM6 cell attachment (data not shown). However, the binding site of laminin-1 for VLA-α3 and α6 integrins is the C-terminal portion (Hall et al, 1990; Tomaselli et al, 1990; Sonnenberg et al, 1991), while YIGSR has been shown to recognize 36-kDa, 38-kDa and 67-kDa cell surface proteins (Graf et al, 1987; Clement et al, 1990) and α4β1 integrin (Maeda et al, 1994). Taken together, these findings indicate that YIGSR may inhibit tumour formation and leukaemic infiltration by competing with laminin for these laminin receptors and/or integrins on leukaemic cells, thus blocking the binding of the cells to basement membrane (Iwamoto et al, 1987). In our study, the growth and dissemination of leukaemic cells were inhibited by the multimeric YIGSR peptide in vivo and in vitro. The precise mechanism of the drug-provoked tumour inhibition is unclear. Although the direct toxicity of YIGSR peptide for leukaemic cells cannot be completely ruled out, Ac-Y16 reduced tumour growth at 0.5–1.0 mg in vitro and selectively inhibited the dissemination of leukaemic cells in vivo. Previous data also suggested that Ac-Y16 is not cytotoxic in vivo (Iwamoto et al, 1996). In the present study, the high dose (1.5–2.0 mg) of Ac-Y16 clearly inhibited the tumour formation and leukaemic infiltration in all peripheral organs, compared with the same dose of Ac-S16, a scrambled multimeric peptide, which showed only a weak inhibitory effect on leukaemic infiltration. Apoptosis of NALM6 cells were induced by Ac-Y16 in cultures, whereas the number of apoptotic cells in the s.c. tumours was not increased by Ac-Y16. Although this discrepancy is not clear, it is possible that the sensitivity of the apoptosis assays may be different between cell cultures and in vivo. Another possibility is that Ac-Y16-mediated apoptosis may occur in much earlier stages after the inoculation of NALM6 cells with Ac-Y16 in SCID mice. Our apoptosis assays were performed at 12 h after the incubation and at this late stage apoptotic cells may not be present and could not be detected. Alternatively, Ac-Y16 may be more potent in inhibiting tumour cell proliferation or inducing necrosis of tumour cells than apoptosis in vivo. In the previous study, the proliferation of HT-1080 cells was markedly decreased by Ac-Y16 at 60–100 µg ml–1, while only a small effect was observed at 30 µg ml–1 (Kim et al, 1994). Proliferation of SW480 cells was reduced at 100 µg ml–1, but had no effect at 30 µg ml–1 (Kim et al, 1994). In our study, the colony formation of leukaemic cells was partially suppressed by Ac-Y16 at 0.5–2.0 mg per 106 cells (5–20 µg ml–1). Although assays used for these two studies are different, their data suggest that the inhibitory activity of Ac-Y16 varies with different cell types. Leukaemic cells usually spread from bone marrow or the tumour burden to peripheral organs as overt leukaemia. In order to disseminate, leukaemic cells enter the circulatory system by crossing the endothelium and the basement membrane. The multimeric YIGSR peptide may inhibit the spreading of leukaemic cells to the vascular endothelium, by blocking leukaemic cell binding to laminin. Only one leukaemic cell line was used in the present study; further analyses is necessary to examine the inhibitory activity of Ac-Y16 for primary leukaemic cells from patients. [1] It has been suggested that the mechanical forces acting on endothelial cells may be sensed in part by cell-matrix connections. We therefore studied the role of different matrix proteins, in particular laminin I, on a shear stress-dependent endothelial response, namely nitric-oxide synthase (eNOS) expression. Primary porcine aortic endothelial cells were seeded onto glass plates either noncoated (NC cells) or precoated with fibronectin (FN cells), laminin (LN cells), or collagen I (CN cells). Western blots were used to detect differences in the final matrix composition of these cells. A shear stress of 16 dyn/cm2 was applied for 6 h. Only LN cells showed detectable amounts of laminin I in their underlying matrix when they reached confluence. They reacted with a 2-fold increase of eNOS expression (n = 16, p < 0.001) to the exposure of shear stress, which went along with enhanced eNOS protein and NO release. In contrast, neither FN cells (n = 9) nor NC cells (n = 13) showed a significant increase of eNOS expression under shear stress. The increase in CN cells was borderline (1.4-fold; n = 9, p < 0.05) and was not associated with an increase of eNOS protein. The shear-induced increase in eNOS expression of LN cells was abolished by the peptide YIGSR, which blocks the cellular binding to laminin I via a 67-kDa laminin-binding protein, whereas a control peptide (YIGSK) had no effect. The induction of eNOS expression by shear stress is stimulated by an interaction of endothelial cells with laminin which is, at least in part, mediated by a 67-kDa laminin-binding protein. [2] |
| 分子式 |
C26H42N8O8
|
|---|---|
| 分子量 |
594.66048
|
| 精确质量 |
594.313
|
| CAS号 |
110590-64-2
|
| PubChem CID |
123977
|
| 序列 |
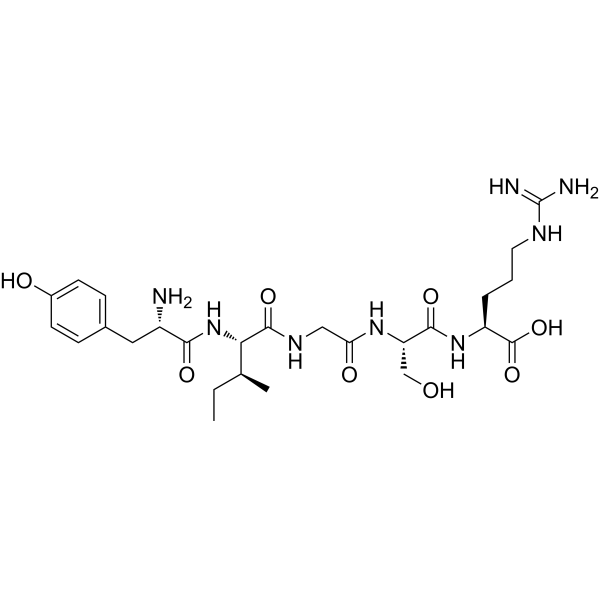
Tyr-Ile-Gly-Ser-Arg;
L-tyrosyl-L-isoleucyl-glycyl-L-seryl-L-arginine
|
| 短序列 |
YIGSR
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.44 g/cm3
|
| 折射率 |
1.633
|
| LogP |
0.673
|
| tPSA |
282.08
|
| 氢键供体(HBD)数目 |
10
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
18
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
937
|
| 定义原子立体中心数目 |
5
|
| SMILES |
CC[C@@H]([C@H](NC([C@@H](N)CC1=CC=C(O)C=C1)=O)C(NCC(N[C@H](C(N[C@H](C(O)=O)CCCNC(N)=N)=O)CO)=O)=O)C
|
| InChi Key |
MWOGMBZGFFZBMK-LJZWMIMPSA-N
|
| InChi Code |
InChI=1S/C26H42N8O8/c1-3-14(2)21(34-22(38)17(27)11-15-6-8-16(36)9-7-15)24(40)31-12-20(37)32-19(13-35)23(39)33-18(25(41)42)5-4-10-30-26(28)29/h6-9,14,17-19,21,35-36H,3-5,10-13,27H2,1-2H3,(H,31,40)(H,32,37)(H,33,39)(H,34,38)(H,41,42)(H4,28,29,30)/t14-,17-,18-,19-,21-/m0/s1
|
| 化学名 |
(2S)-2-[[(2S)-2-[[2-[[(2S,3S)-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid
|
| 别名 |
Tyr-ile-gly-ser-arg; 110590-64-2; YIGSR; H-Tyr-Ile-Gly-Ser-Arg-OH; tyrosyl-isoleucyl-glycyl-seryl-arginine; 120940-31-0; MFCD00076472; (2S)-2-[[(2S)-2-[[2-[[(2S,3S)-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-3-methylpentanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid; YIGSR; Laminin Fragment 929-933;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6816 mL | 8.4082 mL | 16.8163 mL | |
| 5 mM | 0.3363 mL | 1.6816 mL | 3.3633 mL | |
| 10 mM | 0.1682 mL | 0.8408 mL | 1.6816 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。