| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Toll like receptor 7 (TLR7); HSV-1
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:咪喹莫特是一种免疫反应调节剂,可作为 TLR7(Toll 样受体 7)的激动剂。咪喹莫特是一种处方药,用于治疗生殖器疣、浅表基底细胞癌和光化性角化病。 3M 制药部门的科学家发现了该药物,3M 于 1997 年以 Aldara 品牌首次获得 FDA 批准。截至 2015 年,咪喹莫特已成为通用药物,并以多个品牌在全球范围内销售。咪喹莫特通过 TLR7 向免疫系统的先天臂发出信号,通常参与病原体识别。咪喹莫特通过 TLR-7 激活的细胞分泌细胞因子,例如干扰素-α (INF-α)、白介素-6 (IL-6) 和肿瘤坏死因子-α (TNF-α)。咪喹莫特已被研究与派姆单抗联合治疗 IIIB-IV 期黑色素瘤。
|
| 体内研究 (In Vivo) |
咪喹莫特可用于创建牛皮癣动物模型。咪喹莫特通过 NK 细胞活化、活化巨噬细胞分泌一氧化氮和细胞因子以及 B 淋巴细胞分化和增殖来激发动物模型中的先天免疫反应。通过触发、产生和释放白细胞介素 (IL)-6、肿瘤坏死因子 (TNF)-α 和干扰素-α (IFN-α) 等细胞因子,咪喹莫特可激活先天免疫反应 [1]。
imiquimod咪喹莫特诱导小鼠银屑病样皮肤炎症[5] 8至11周龄的小鼠在剃毛的背部和右耳连续5或6天每天局部服用62.5mg市售的imiquimod/咪喹莫特/IMQ乳膏(5%),相当于每天3.125mg的活性化合物。该剂量是根据经验确定的,可在小鼠中引起最佳和可重复的皮肤炎症(数据未显示)。对照组小鼠用对照溶媒乳膏进行类似处理。 相对于IMQ应用的开始,在第-3、0和3天向小鼠注射400μg/小鼠-大鼠抗小鼠CD3单克隆抗体17A2会耗尽CD3+细胞。 皮肤炎症诱导和症状逆转评估[6] 用imiquimod/咪喹莫特治疗后,动物分为两组:IMQ组-用于第7天咪喹莫特效应的组织学评估,BTM组-用于评估imiquimod咪喹莫特/IMQ诱导的变化逆转,通过计算倍他米松软膏(0.5mg/g)治疗前后的PASI来确定。实验设计如图1所示。 PASI评估皮肤炎症[6] BTM组在第0天(应用咪喹莫特前)、第7天(应用米喹莫特一段时间后)和第11天通过采用银屑病面积严重程度指数(PASI)计算对大鼠皮肤病变进行评估。使用银屑病评分表对PASI评估的三个指标(红斑、鳞屑和增厚)进行单独评分。由于实验区域的大小没有差异,因此没有考虑区域得分。分数分配如下:0--无;1--轻微;2--中等;3--标记,4--非常标记。累积得分作为炎症严重程度的衡量标准(最高得分为12)。 咪喹莫特(IMQ)是一种TLR7/8配体和强效免疫激活剂,局部应用可诱导和加剧银屑病,这是一种慢性炎症性皮肤病。最近,IL-23/IL-17轴在银屑病中起着至关重要的作用。我们假设IMQ诱导的小鼠皮炎可以作为分析银屑病样皮炎致病机制的模型,并评估其IL-23/IL-17轴依赖性。每日在小鼠背部皮肤上应用IMQ,诱导类似斑块型银屑病的炎症鳞状皮肤病变。这些病变表现为表皮增殖增加、分化异常、表皮微泡中性粒细胞积聚、新生血管生成以及由CD4(+)T细胞、CD11c(+)树突状细胞和浆细胞样树突状细胞组成的浸润。IMQ诱导表皮IL-23、IL-17A和IL-17F的表达,以及脾脏Th17细胞的增加。IMQ诱导的皮炎部分依赖于T细胞的存在,而在IL-23或IL-17受体缺乏的小鼠中,疾病的发展几乎完全被阻断,这表明IL-23/IL-17轴起着关键作用。总之,仅应用天然TLR7/8配体IMQ可迅速诱导与人类银屑病非常相似的皮炎,严重依赖IL-23/IL-17轴。这种快速方便的模型可以进一步阐明银屑病的致病机制并评估新的治疗方法。[5] 咪喹莫特(IMQ)诱导的小鼠类人银屑病已被证明在测试和开发新疗法方面是有效的。IMQ银屑病模型已成为广泛使用的动物模型,然而,它在不同大鼠品系中的特征并不完全。我们的目的是在宏观、组织学、遗传学以及细胞因子和趋化因子激活水平上评估IMQ和倍他米松治疗诱导和逆转银屑病病变的效果。Wistar大鼠局部用IMQ治疗。采用银屑病面积严重程度指数(PASI)在基线时、IMQ症状诱导后和倍他米松症状逆转后计算。研究了血浆中细胞因子和趋化因子水平的系统性影响。进行皮肤活检以评估组织学症状和选定的炎性细胞因子和受体基因表达水平。倍他米松治疗后,皮肤病变的逆转具有显著性(p=0.03)。未经治疗和IMQ治疗的皮肤在某些标志物上存在显著的组织学差异(p<0.05),尽管倍他米松治疗没有显著降低。14个基因在IMQ后显著上调,4个基因在倍他米松逆转皮肤病变后下调。这项工作为咪喹莫特诱导的银屑病的生物学效应及其在Wistar大鼠中被倍他米松治疗逆转提供了新的见解。它还有助于了解大鼠模型用于测试新型抗银屑病药物的一般知识。[6] |
| 酶活实验 |
siRNA介导的基因表达抑制。[3]
使用对人胱抑素A特异的小干扰RNA(siRNA)、对人腺苷受体A1特异的siRNA[腺苷A1-R siRNA(h)]和对照siRNA。根据制造商的说明,使用FUGENE 6试剂将siRNA(0.5μg)转染到细胞中。培养24小时后,一些(但不是全部)细胞用10μg的imiquimod/咪喹莫特处理24小时或不处理24小时。然后用MOI为1的HSV-1 VR3感染细胞。如上所述测定培养上清液中的病毒滴度。 通过竞争试验确定与腺苷受体的结合。[3] 3H标记的DPCPX是腺苷A1受体的选择性拮抗剂,购自Perkin-Elmer。将CHO-A1或CHO-K1细胞以每孔2×103个细胞的密度接种到96孔板中,然后孵育过夜。将细胞与[3H]DPCPX(550 Bq/孔)和竞争化学物质(咪喹莫特和瑞喹莫特[各为100μM]或DPCPX[1μM])在含有10%FBS和青霉素-链霉素的IMDM中在CO2培养箱中孵育20分钟。孵育后,用PBS(−)洗涤细胞四次,并用裂解缓冲液(1%NP-40、5%甘油、5 mM EDTA、100 mM NaCl、50 mM HEPES[pH 7.4])裂解。使用LS6500液体闪烁计数器 测量裂解物中[3H]DPCPX的每分钟计数(cpm)。[3H]DPCPX在CHO-A1细胞腺苷受体A1上的选择性结合量是通过从作为对照的CHO-K1细胞的cpm值中减去CHO-A1的cpm来计算的。没有竞争对手的cpm值设置为100%。 咪喹莫特/imiquimod是咪唑喹啉家族的一种小分子免疫反应调节剂,在体外和体内临床应用中均显示出显著的抗肿瘤和抗病毒功效。已经证明,这种活性是通过Toll样受体(TLR)7-和TLR8信号级联介导的,导致促炎细胞因子的分泌,并连续诱导肿瘤导向的细胞免疫反应。此外,咪喹莫特在肿瘤细胞中具有直接的促凋亡活性。我们在这里证明,咪喹莫特在没有TLR7和TLR8的情况下诱导转录因子NF-κB的激活和促炎细胞因子的下游产生。在用人腺苷受体亚型稳定转染的中国仓鼠卵巢细胞中,我们在放射性配体结合竞争实验中表明,咪喹莫特在临床环境中相关的浓度下与腺苷受体结合,与A(1)和A(2A)亚型的亲和力最高。还研究了咪喹莫特对腺苷环化酶受体介导激活的影响,这些实验表明咪喹莫特是腺苷受体拮抗剂。此外,咪喹莫特对受体下游的腺苷酸环化酶活性具有抑制作用。最后,使用转化的人角质形成细胞,我们提供了实验证据,表明咪喹莫特和A(2A)腺苷受体特异性化合物在没有免疫细胞的情况下同样诱导促炎细胞因子。因此,咪喹莫特似乎通过拮抗腺苷受体依赖性cAMP增加和伴随的受体非依赖性cAMP产生抑制来抑制炎症的重要反馈机制。这些新机制可能与促炎细胞因子的积极诱导协同作用,至少可以部分解释一些患者体内观察到的严重炎症。 |
| 细胞实验 |
感染实验和细胞活力测定。[3]
FL细胞以105个细胞/ml的浓度接种在6-cm的培养皿中,培养24小时。为了分析imiquimod/咪喹莫特对细胞存活率和病毒复制的影响,在病毒感染前,将细胞在含有咪喹莫特的培养基中培养12或24小时。然后以0.1或1的感染倍数(MOI)用HSV-1接种细胞,孵育1小时以吸收,然后再培养24小时。如前所述,使用Vero细胞通过空斑试验测定上清液中的病毒滴度。使用细胞计数试剂盒8 通过改良的3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑测定法测定细胞存活率。 微阵列分析。[3] 从FL细胞中制备总细胞RNA,这些细胞在10μg/ml的浓度下与或不与imiquimod/咪喹莫特一起培养24小时。使用3D Gene Human OligoChip 25K进行微阵列分析。 |
| 动物实验 |
Tissue ELISA revealed that imiquimod specifically reduced IL-1β and IL-6 secretion in the treated mouse paws (Panels F-G), whereas neutrophil infiltration (visualized by MPO quantification, Panel H) and TNF-α production (panel I) remained unaffected. RTqPCR and western blot analysis further documented the negative effect of imiquimod on the transcription of the Il-1β and Il-6 genes (Panels J-K) and IL-1β pro-form expression (Panel L).
Imiquimod-induced psoriasis-like skin inflammation in mice [5] Mice at 8 to 11 wk of age received a daily topical dose of 62.5 mg of commercially available imiquimod/IMQ cream (5%) on the shaved back and the right ear for 5 or 6 consecutive days, translating in a daily dose of 3.125 mg of the active compound. This dose was empirically determined to cause most optimal and reproducible skin inflammation in mice (data not shown). Control mice were treated similarly with a control vehicle cream. CD3+ cells were depleted by injection of the mice with 400 μg/mouse rat-anti-mouse CD3 mAb 17A2 on days −3, 0, and 3, relative to the start of IMQ application. Skin inflammation induction and symptom reversal evaluation [6] Following the treatment with imiquimod, animals were divided into two groups: IMQ Group—for the histological evaluation of imiquimod effects on Day 7, and BTM Group—for evaluation of imiquimod/IMQ induced changes reversal determined by calculation of PASI prior and after the treatment with betamethasone ointment (0.5 mg/g). Experimental design is provided in Fig. 1. PASI evaluation of skin inflammation [6] The evaluation of rat skin lesions was performed by adopted Psoriasis Area Severity Index (PASI) calculation on day 0 (before imiquimod application), day 7 (after period of imiquimod application) and day 11 in BTM group. PASI evaluation was scored individually for three indicators (erythema, scaling, and thickening) using psoriasis scoring tables. As the size of experimental area did not differ, the area score was not taken into account. Scores were assigned as follows: 0—none; 1—slight; 2—moderate; 3—marked and 4—very marked. The cumulative score served as a measure of the inflammation severity (maximum score of 12). |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed through skin (as a cream) Following topical application to the skin in adults with actinic keratosis (75-mg doses 3 times weekly for 16 weeks), 0.08-0.15% of the dose is eliminated in urine as unchanged drug and metabolites. Following topical application in patients with HPV warts, 0.11 or 2.41% of the dose is eliminated in urine as unchanged drug and metabolites in men or women, respectively. Imiquimod is absorbed systemically following topical application to skin. In adults with actinic keratosis who received topical imiquimod 5% cream 3 times weekly for 16 weeks, mean peak serum concentrations at the end of week 16 were approximately 0.1, 0.2, or 3.5 ng/mL in those treated on the face (12.5-mg doses), scalp (25-mg doses), or hands/arms (75-mg doses), respectively. Systemic exposure appeared to depend more on the surface area of the application site than on the total applied dose. In patients with external genital and perianal human papillomavirus (HPV) warts who received topical imiquimod 5% cream (average dose 4.6 mg), mean peak serum concentrations were 0.4 ng/mL. Biological Half-Life 20 hours (topical dose), 2 hours (subcutaneous dose) Studies using subcutaneous imiquimod indicate the drug has an apparent half-life of 2 hours. Following topical application, imiquimod appears to be retained in the skin for prolonged periods since the half-life is approximately 10 times greater than that reported following subcutaneous administration. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of imiquimod during breastfeeding. However, because the drug is used on a limited surface area and poorly absorbed into the maternal circulation, effects on the infant are unlikely. If imiquimod is required by the mother, it is not a reason to discontinue breastfeeding. Do not apply imiquimod to the breast or nipple and ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
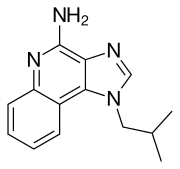
Adjuvants, Immunologic; Antineoplastic Agents Imiquimod is used topically for the treatment of clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratosis on the face or scalp in immunocompetent adults; treatment of biopsy-confirmed, primary superficial basal cell carcinoma in immunocompetent adults; and treatment of external genital and perianal exophytic warts (condylomata acuminata) caused by human papillomavirus (HPV). /Included in US product label/ Topical imiquimod has been effective when used in a limited number of adults and children for the treatment of molluscum contagiosum. /NOT included in US product label/ Imiquimod 5% cream has been used for the topical treatment of external genital and perianal HPV warts in a limited number of adults with human immunodeficiency virus (HIV) infection; however, the response rate appears to be lower in these individuals than in those who are not HIV infected. /NOT included in US product label/ For more Therapeutic Uses (Complete) data for Imiquimod (6 total), please visit the HSDB record page. Drug Warnings Adverse local reactions, including erythema, erosion, excoriation/flaking, and edema, commonly occur at the site of application of imiquimod and/or surrounding areas. These reactions usually are mild to moderate in severity; however, severe local reactions have been reported. In controlled studies in adults with actinic keratosis, the most frequently reported local skin reactions in those receiving imiquimod 5% cream (twice weekly for 16 weeks) were erythema (97%), flaking/scaling/dryness (93%), scabbing/crusting (79%), edema (49%), erosion/ulceration (48%), weeping/exudate (22%), and vesicles (9%).1 Application site reactions (e.g., bleeding, burning, induration, irritation, pain, pruritus, stinging, tenderness) occurred in 33% of those receiving topical imiquimod compared with 14% of those receiving placebo. In these studies, 16% of patients discontinued imiquimod treatment because of local or application site reactions and 91% of these were able to resume treatment after a rest period. Adverse dermatologic reactions at sites away from the site of application have been reported in some patients receiving topical imiquimod. Remote site reactions have included bleeding, burning, edema, erosion, erythema, excoriation/flaking, induration, pain, pruritus, tenderness, tinea cruris, and ulceration. When imiquimod 5% cream was used in controlled studies in patients with genital and perianal HPV warts (3 times weekly for up to 16 weeks), erythema occurred in 58-65%, erosion in 30-31%, excoriation/flaking in 18-26%, edema in 12-18%, scabbing in 4-13%, induration in 5-7%, ulceration in 4-8%, and vesicles in 2-3% of those receiving the drug.1 In addition, application site reactions in those receiving the drug included pruritus (22-32%), burning (9-26%), pain (2-8%), and soreness (0-3%). In addition, fungal infections occurred in 2-11% of patients receiving the drug. Overall, 1.2% of patients in these studies discontinued treatment because of local or application site reactions. For more Drug Warnings (Complete) data for Imiquimod (25 total), please visit the HSDB record page. Pharmacodynamics Imiquimod is an immune response modifier that acts as a toll-like receptor 7 agonist. Imiquimod is commonly used topically to treat warts on the skin of the genital and anal areas. Imiquimod does not cure warts, and new warts may appear during treatment. Imiquimod does not fight the viruses that cause warts directly, however, it does help to relieve and control wart production. It is not used on warts inside the vagina, penis, or rectum. Imiquimod is also used to treat a skin condition of the face and scalp called actinic keratoses. Imiquimod can also be used to treat certain types of skin cancer called superficial basal cell carcinoma. Imiquimod is particularly useful on areas where surgery or other treatments may be difficult, complicated or otherwise undesirable, especially the face and lower legs. Imiquimod is an imidazoquinoline fused [4,5-c] carrying isobutyl and amino substituents at N-1 and C-4 respectively. A prescription medication, it acts as an immune response modifier and is used to treat genital warts, superficial basal cell carcinoma, and actinic keratosis. It has a role as an antineoplastic agent and an interferon inducer. Imiquimod is a prescription medicine approved by the U.S. Food and Drug Administration (FDA). It is a cream for topical use only. Imiquimod is FDA-approved for the treatment of certain skin conditions, including: Actinic keratosis (a skin condition that may develop into skin cancer) External genital warts (warts on the outside of the genitals) and perianal warts (warts around the outside of the anus) External genital and perianal warts are caused by the human papillomavirus (HPV). HPV can be an opportunistic infection (OI) of HIV. Imiquimod is an immune response modifier that acts as a toll-like receptor 7 agonist. Imiquimod is commonly used topically to treat warts on the skin of the genital and anal areas. Imiquimod does not cure warts, and new warts may appear during treatment. Imiquimod does not fight the viruses that cause warts directly, however, it does help to relieve and control wart production. Miquimod is also used to treat a skin condition of the face and scalp called actinic keratoses and certain types of skin cancer called superficial basal cell carcinoma. The mechanism of action of imiquimod is as an Interferon Inducer. The physiologic effect of imiquimod is by means of Increased Cytokine Activity, and Increased Cytokine Production. Imiquimod is a synthetic agent with immune response modifying activity. As an immune response modifier (IRM), imiquimod stimulates cytokine production, especially interferon production, and exhibits antitumor activity, particularly against cutaneous cancers. Imiquimod's proapoptotic activity appears to be related to Bcl-2 overexpression in susceptible tumor cells. (NCI04) IMIQUIMOD is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 1997 and has 6 approved and 47 investigational indications. This drug has a black box warning from the FDA. A topically-applied aminoquinoline immune modulator that induces interferon production. It is used in the treatment of external genital and perianal warts, superficial CARCINOMA, BASAL CELL; and ACTINIC KERATOSIS. |
| 分子式 |
C14H16N4
|
|---|---|
| 分子量 |
240.3
|
| 精确质量 |
240.137
|
| 元素分析 |
C, 69.97; H, 6.71; N, 23.32
|
| CAS号 |
99011-02-6
|
| 相关CAS号 |
Imiquimod hydrochloride;99011-78-6;Imiquimod maleate;896106-16-4;Imiquimod-d6;Imiquimod-d9;2712126-48-0
|
| PubChem CID |
57469
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
456.7±48.0 °C at 760 mmHg
|
| 熔点 |
292-294°C
|
| 闪点 |
230.0±29.6 °C
|
| 蒸汽压 |
0.0±1.1 mmHg at 25°C
|
| 折射率 |
1.681
|
| LogP |
3.46
|
| tPSA |
56.73
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
294
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
DOUYETYNHWVLEO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H16N4/c1-9(2)7-18-8-16-12-13(18)10-5-3-4-6-11(10)17-14(12)15/h3-6,8-9H,7H2,1-2H3,(H2,15,17)
|
| 化学名 |
1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine
|
| 别名 |
S26308; R 837; TMX-101; S 26308; R837; TMX 101; S-26308; R837; TMX101; 99011-02-6; 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine; 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine; Beselna; 9050-31-1; Brand names: Aldara; Zyclara; Vyloma; Beselna.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1615 mL | 20.8073 mL | 41.6146 mL | |
| 5 mM | 0.8323 mL | 4.1615 mL | 8.3229 mL | |
| 10 mM | 0.4161 mL | 2.0807 mL | 4.1615 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。