| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 1g |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The duration of effects in humans compared to THC seems to be shorter for JWH-018 (1-2 hours) and considerably longer for CP 47,497-C8 (5-6 hours), as reported in a self-experiment. Metabolism / Metabolites ... The high pharmacological and addictive potency of JWH-018 highlights the importance of elucidating the metabolism of JWH-018, without which a meaningful insight into its pharmacokinetics and its toxicity would not be possible. In the present study, the cytochrome P450 phase I metabolites of JWH-018 were investigated, after in vitro incubation of the drug with human liver microsomes, followed by liquid chromatography-tandem mass spectrometry analysis. This revealed monohydroxylation of the naphthalene ring system, the indole moiety, and the alkyl side chain. In addition, observations were made of dihydroxylation of the naphthalene ring system, and the indole moiety, or as result of a combination of monohydroxylations of both the naphthalene ring system and the indole moiety or the alkyl side chain, or a combination of monohydroxylations of both the indole ring system and the alkyl side chain. There is also evidence of trihydroxylation at different locations of the hydroxyl groups in the molecule. Furthermore, dehydration of the alkyl side chain, in combination with both monohydroxylation and dihydroxylation as well as arene oxidation of the naphthalene ring system, combined with both monohydroxylation and dihydroxylation at different sites of oxidation were found. N-dealkylation also in combination with both monohydroxylation and dihydrodiol formation of the N-dealkylated metabolite was detected. Finally, a metabolite was found carboxylated at the alkyl side chain. ... This study evaluates nine human recombinant uridine diphosphate-glucuronosyltransferase (UGT) isoforms and human liver and intestinal microsomes for their ability to glucuronidate hydroxylated metabolites of 1-naphthalenyl-1(1-pentyl-1H-indol-3-yl)-methanone (JWH-018) and (1-butyl-1H-indol-3-yl)-1-naphthalenyl-methanone (JWH-073), the two most common synthetic cannabinoids found in K2 products. Conjugates were identified and characterized using liquid chromatography/tandem mass spectrometry, whereas kinetic parameters were quantified using high-performance liquid chromatography-UV-visible methods. UGT1A1, UGT1A3, UGT1A9, UGT1A10, and UGT2B7 were shown to be the major enzymes involved, showing relatively high affinity with K(m) ranging from 12 to 18 uM for some hydroxylated K2s. These UGTs also exhibited a high metabolic capacity for these compounds, which indicates that K2 metabolites may be rapidly glucuronidated and eliminated from the body. ... The aim of this study was to elucidate the metabolism of JWH-018. An ethanolic extract was prepared from an incense containing large amounts of JWH-018. After removal of the ethanol, the residue was given to Wistar rats by gastric intubation and urine was collected over 24 hours. For identification, the metabolites were isolated after enzymatic or acidic cleavage of conjugates by liquid-liquid extraction (LLE) or solid-phase extraction (C18) followed by acetylation. The metabolites were separated and identified by GC and MS in the electron ionization (EI) mode. The parent compound JWH-018 could be found in the urine extracts only in small amounts. Besides the parent compound, the N-dealkylated metabolite could be detected in urine in small amounts. The highest signals could be observed for the hydroxylated N-dealkyl metabolites. Hydroxylation can take place in both aromatic systems, the naphthalene and the indole part, which could be shown by mass shift of the corresponding fragments. JWH-018 is extensively metabolized in rats. According to our experience similar metabolic patterns can be expected in humans. ... ... It is worth nothing that unlike metabolites of most synthetic cannabinoids, JWH-018 hydroxylated metabolites retain in vitro and in vivo activity at CB1 receptors... For more Metabolism/Metabolites (Complete) data for 1-pentyl-3-(1-naphthoyl) indole (9 total), please visit the HSDB record page. |
|---|---|
| 参考文献 |
Thaxton-Weissenfluh A, Alsegiani AS, Abiedalla Y, DeRuiter J, Smith F, Clark CR. Analytical studies on the 2-naphthoyl substituted-1-n-pentylindoles: Regioisomeric synthetic cannabinoids. J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Mar 1;1077-1078:77-84. doi: 10.1016/j.jchromb.2018.01.036. Epub 2018 Jan 31. PubMed PMID: 29413581.
|
| 其他信息 |
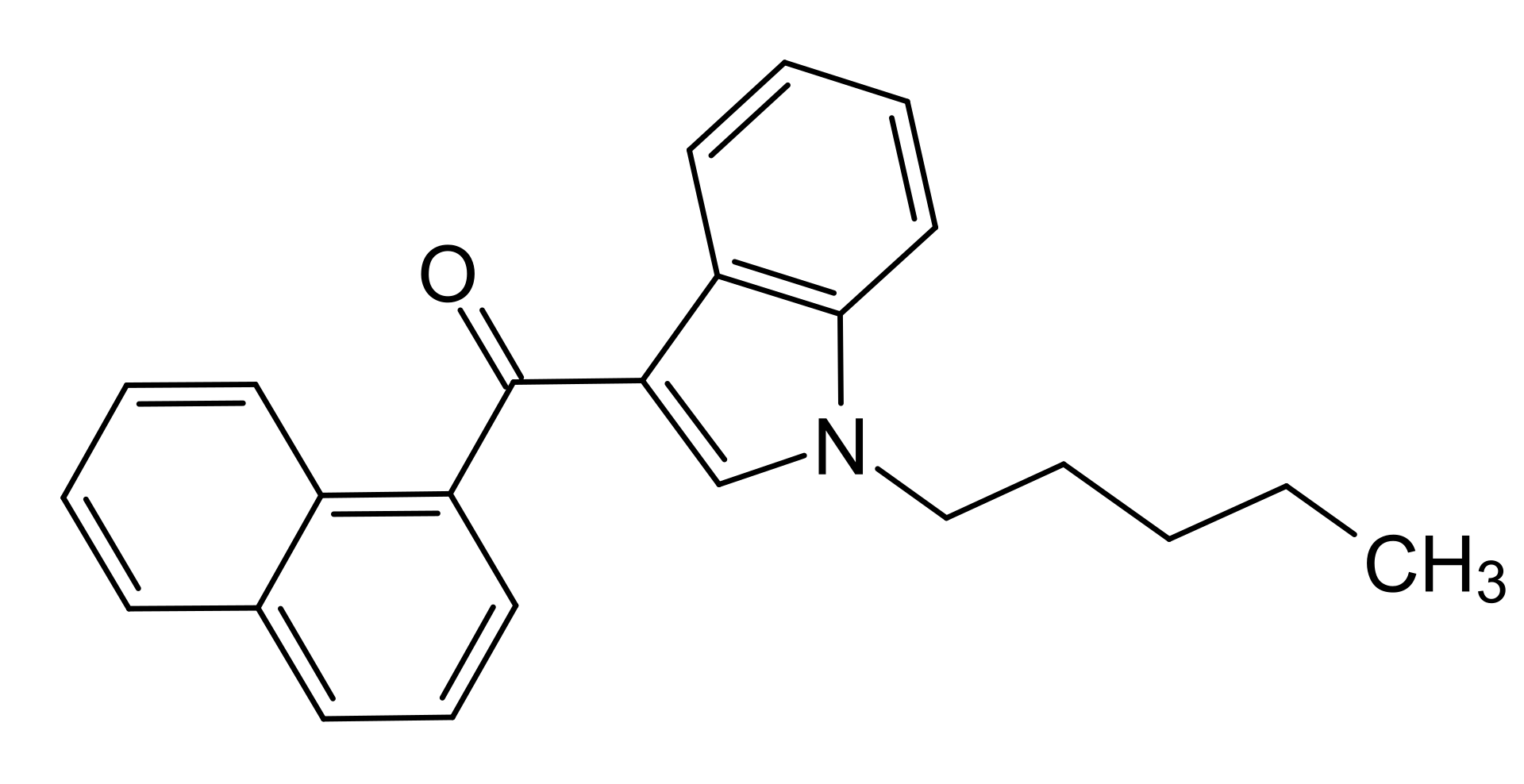
JWH 018 is an indolecarboxamide.
1-pentyl-3-(1-naphthoyl)indole is a DEA Schedule I controlled substance. Substances in the DEA Schedule I have no currently accepted medical use in the United States, a lack of accepted safety for use under medical supervision, and a high potential for abuse. It is a Cannabimimetic agents substance. Mechanism of Action ... JWH-018 is probably the most studied synthetic cannabinoid. It has been found in multiple preparations and has been shown to be a potent cannabinoid receptor (CB) agonist. CB1 is the principal receptor thought to be most highly responsible for the euphoria and psychoactive effects of THC. The CB2 receptor resides mostly in the immune system but has some effect on pain control and mood regulation. ... /Synthetic cannabinoid/ agonistic activity on the CB1 receptor is responsible for elevating mood and inducing a feeling of well-being. Some /synthetic cannabinoid/ users have reported effects similar to or even stronger than those obtained by smoking cannabis, such as physical relaxation, changes in perception, and mild euphoria. The higher potency of action of these synthetic cannabinoids might be explained by in vitro experiments that have suggested that, while THC acts as a partial agonist on the CB1 receptor, JWH-018 acts as a full and potent agonist. Moreover, compared with THC, JWH-018 possesses approximately a fourfold higher affinity to the cannabinoid CB1 receptor and 10-fold higher affinity to CB2 receptor. ... JWH-018 (naphthalen-1-yl-(1-pentylindol-3-yl)methanone) was first synthesized during an analysis aiming at developing new cannabimimetic indole compounds with potential therapeutic effects comparable with those of THC. It belongs to the aminoalkylindole family and has been shown to have a binding affinity for the CB1 receptors in the low nanomolar range (approximately 9 nM). In cannabinoid receptor expressing CHO cells, JWH-018 inhibits forskolin-stimulated cAMP production, whereas in HEK293 cells stably expressing this receptor, it was recently found to activate multiple cannabinoid receptor signaling pathways, including the phosphorylation of ERK1/2 mitogen activated protein kinase and the internalization of CB1 receptors. JWH-018 dose-dependently inhibits glutamate release in autaptic excitatory hippocampal neurons, probably acting on the CB1 receptor, an effect reversed by administration of the CB1 receptor antagonist rimonabant. In vivo studies showing that JWH-018 induces analgesia, catalepsy, hypomotility, and hypothermia, namely the tetrad of behaviors classically caused by cannabinoids administration, have confirmed that this compound acts as a potent and effective CB1 receptor agonist. Specifically, JWH-018 displayed fourfold affinity to the CB1 receptor and about 10-fold affinity to the CB2 receptor compared with THC. It is worth nothing that unlike metabolites of most synthetic cannabinoids, JWH-018 hydroxylated metabolites retain in vitro and in vivo activity at CB1 receptors, a finding that in conjunction with the higher CB1 receptor affinity and activity relative to THC may contribute to the greater prevalence of adverse effects observed with JWH-018-containing products relative to marijuana. |
| 分子式 |
C24H23NO
|
|---|---|
| 分子量 |
341.44
|
| 精确质量 |
341.177
|
| CAS号 |
209414-07-3
|
| PubChem CID |
10382701
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 熔点 |
54-60 °C
|
| LogP |
6.3
|
| tPSA |
22
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
475
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
JDNLPKCAXICMBW-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H23NO/c1-2-3-8-16-25-17-22(20-13-6-7-15-23(20)25)24(26)21-14-9-11-18-10-4-5-12-19(18)21/h4-7,9-15,17H,2-3,8,16H2,1H3
|
| 化学名 |
1-Pentyl-3-(1-naphthoyl) indole
|
| 别名 |
AM-678 AM678 AM 678 JWH018 JWH 018 JWH-018
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9288 mL | 14.6439 mL | 29.2877 mL | |
| 5 mM | 0.5858 mL | 2.9288 mL | 5.8575 mL | |
| 10 mM | 0.2929 mL | 1.4644 mL | 2.9288 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。