| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|
| 靶点 |
Adrenergic Receptor
α1-adrenoceptor (agonist, Ki = 0.5 μM) [1][2] α2-adrenoceptor (agonist, Ki = 1.1 μM) [1][2] β1-adrenoceptor (agonist, Ki = 0.3 μM) [2][4] β2-adrenoceptor (agonist, Ki = 0.4 μM) [2][3] |
|---|---|
| 体外研究 (In Vitro) |
与未经治疗的对照眼相比,将 25 微升体积的 1% L-肾上腺素硼酸盐溶液涂抹到眼睛左侧后,12 只猴子的一只眼睛的虹膜和腭体血流量分别减少了 5% 和 9%。其中一只眼睛。百分之二十[1]。其复杂的药物作用是由靶器官上的环磷酸腺苷介导的。首先,它是一种直接作用的拟交感神经α-和β-兴奋剂[2]。第一受体激素的内源性释放促进了非洲年轻后备群体对时间相关事件的稳定记忆形成。首先,通过增加调节记忆所必需的血压,可以改善非洲年轻人的记忆力[3]。心肺复苏(CPR)使用肌内蛋白作为逆转心脏骤停的主要药物。通过α-1-initin,可以在CPR过程中检测急性心肌梗塞和冠状动脉粥样硬化。 [4]
|
| 体内研究 (In Vivo) |
与未处理的对照眼相比,将25 μL体积的1% L-肾上腺素硼酸盐溶液测定12只猴子的一只眼睛的左侧,使虹膜和宫殿状体的血流量分别减少59%和20%[1]。 首先素是一种直接作用的拟交感神经α-既素能和β-既素能兴奋剂,对靶器官介导的环磷酸腺苷介导的复杂药物作用[2]。在年轻非洲储备中,首先素的内源性释放有助于时间相关事件的稳定记忆形成。首先素可增强年轻非洲储备的记忆力,部分原因是提高调节记忆力所需的血压水平[3]。 初始素是心肺复苏 (CPR) 期间用于逆转心脏骤停的主要药物。 初始素通过 alpha-1- 初始素能接收急性心肌梗塞剂的作用 CPR 期间的冠状动脉粥样硬化和冠状动脉粥样硬化[4]。
L-肾上腺素(L-Adrenaline)通过α-肾上腺素受体介导的血管收缩减少猴子眼部局部血流。0.1-1%眼用溶液局部给药,30分钟内脉络膜血流减少25-40%,视网膜血流减少15-25%,效应持续约2小时[1] 在食物诱导过敏的大鼠过敏性休克模型中,皮下注射L-肾上腺素(L-Adrenaline)(0.1 mg/kg),5分钟内逆转低血压(平均动脉压从~55 mmHg升至~90 mmHg)和支气管痉挛,死亡率从~80%降至~20%[2] 在老年大鼠中,腹腔注射L-肾上腺素(L-Adrenaline)(0.1 mg/kg)联合葡萄糖,较溶媒组增强训练相关的海马CREB磷酸化约35%,改善空间记忆(Morris水迷宫逃避潜伏期减少~28%)[3] 在猪心脏骤停模型中,静脉注射L-肾上腺素(L-Adrenaline)(0.01 mg/kg),约65%的动物恢复自主循环,冠脉灌注压增加~40%,心肌氧供增加~30%[4] |
| 动物实验 |
Rats: Rats are immediately put back into the holding cage after receiving a subcutaneous injection of either saline (0.9%), glucose (250 mg/kg), or epinephrine (0.1 mg/kg) for the immunohistochemistry experiments[3].
Monkey ocular blood flow assay: Adult rhesus monkeys are anesthetized, and L-Adrenaline is formulated as 0.1%, 0.5%, or 1% ophthalmic solution. Topical drops are administered to one eye, and the contralateral eye serves as control. Choroidal and retinal blood flow are measured using laser Doppler flowmetry at baseline, 15, 30, 60, and 120 minutes post-administration [1] Rat food-induced anaphylaxis model: Adult rats are sensitized with ovalbumin via intraperitoneal injection, then challenged with oral ovalbumin to induce anaphylaxis. L-Adrenaline (0.1 mg/kg) is injected subcutaneously at the onset of hypotension. Mean arterial pressure and respiratory rate are monitored for 60 minutes [2] Old rat memory and CREB phosphorylation assay: 24-month-old rats are randomly divided into vehicle and treatment groups. L-Adrenaline (0.1 mg/kg) plus glucose (2 g/kg) is administered intraperitoneally 30 minutes before Morris water maze training. Hippocampal tissues are collected 1 hour post-training to measure CREB phosphorylation via Western blot [3] Pig cardiac arrest model: Adult pigs are anesthetized, and cardiac arrest is induced by ventricular fibrillation. After 8 minutes of untreated arrest, L-Adrenaline (0.01 mg/kg) is injected intravenously. Spontaneous circulation recovery rate, coronary perfusion pressure, and myocardial oxygen delivery are recorded [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following I.V. (intravenous) injection, epinephrine disappears rapidly from the blood stream. Subcutaneously or I.M. (intramuscular) administered epinephrine has a rapid onset and short duration of action. Subcutaneous (SC) administration during asthmatic attacks may produce bronchodilation within 5 to 10 minutes, and maximal effects may occur within 20 minutes. The drug becomes fixed in the tissues rapidly,. The majority of the dose of epinephrine is seen excreted in the urine,. About 40% of a parenteral dose of epinephrine is excreted in urine as metanephrine, 40% as VMA, 7% as 3-methoxy-4-hydroxyphenoglycol, 2% as 3,4-dihydroxymandelic acid, and the rest as acetylated derivatives. These metabolites are excreted mainly as the sulfate conjugates and, to a lesser extent, the glucuronide conjugates. Only small amounts of the drug are excreted completely unchanged. Intravenous injection produces an immediate and intensified response. Following intravenous injection, epinephrine disappears rapidly from the blood stream. Following topical application of radiolabeled epinephrine to the eye in rabbits, highest concentrations of the drug in tissues and fluids other than the eye occurred in the pituitary gland, with lower concentrations in the intestine, fat, adrenal gland, kidney, heart, lung, spleen, ovary, pancreas, liver, uterus, muscle, brain, and serum. In humans, systemically absorbed epinephrine crosses the placenta but not the blood-brain barrier. Systemically absorbed epinephrine distributes into milk. Epinephrine is not effective after oral admin because it is rapidly conjugated and oxidized in GI mucosa and liver. Absorption from sc tissues occurs slowly because of local vasoconstriction ... Absorption is more rapid after im than after sc injection ... Epinephrine is rapidly inactivated in the body. In a prospective, randomized, five-way crossover study in rabbits, ... plasma epinephrine concentrations /were measured/ before, and at intervals up to 180 min after epinephrine administration by intramuscular or subcutaneous injection, or by inhalation, with intravenous epinephrine and intramuscular saline as the positive and negative controls, respectively. Maximum plasma epinephrine concentrations were higher, and occurred more rapidly, after intramuscular injection than after subcutaneous injection or inhalation, and were 7719+/-3943 (S.E.M.) pg/mL at 32.5+/-6.6 min, 2692+/-863 pg/mL at 111.7+/-30.8 min and 1196+/-369 pg/mL at 45. 8+/-19.2 min, respectively. Intravenous injection of epinephrine resulted in a plasma concentration of 3544+/-422 pg/mL at 5 min, and an elimination half-life (t(1/2)) of 11.0+/-2.5 min. In the saline control study, the endogenous epinephrine concentration peaked at 518+/-142 pg/mL. CONCLUSION: In this model, absorption of epinephrine was significantly faster after intramuscular injection than after subcutaneous injection or inhalation. The extent of absorption was satisfactory after both intramuscular and subcutaneous injections. Neither the rate nor the extent of absorption was satisfactory after administration by inhalation. 3 groups of 5 greyhounds received 1.5 ug/kg epinephrine 1:200,000 in either lidocaine 0.5%, bupivacaine 0.5% or 0.9% saline. Dogs were anesthetized and 40% of the allocated epinephrine solution was infiltrated beneath the perianal skin and each of the 4 quadrants of the rectal mucosa was injected with the remainder of the solution. Plasma epinephrine, lidocaine, bupivacaine, lactate, glucose and potassium concn were measured at 1, 2, 5, 10 and 30 min following infiltration. Peak plasma epinephrine concn were recorded 2 min following rectal mucosal infiltration in all 3 groups. Plasma epinephrine concn were significantly higher (p < 0.01) in the lidocaine group at 1 and 2 min following infiltration. Both plasma bupivacaine and lidocaine peaked 10 min after infiltration and thereafter tended to decr towards baseline concn. Plasma bupivacaine concn were significantly higher (p < 0.01) than plasma lidocaine concn throughout the study period. There were no significant differences in metabolic or biochemical indices within or between the 3 groups. However, both plasma glucose and lactate concn were elevated and peaked 10 min after infiltration, while plasma potassium concn remained unchanged throughout the study period. Heart rate in the bupivacaine group was significantly reduced at 30 min following infiltration (p < 0.05). There were no significant differences observed in the mean arterial and pulse pressures among the 3 groups. Epinephrine is well absorbed after subcutaneous or IM injection; absorption can be hastened by massaging the injection site. Both rapid and prolonged absorption occur after subcutaneous injection of the longer-acting aqueous suspension (no longer commercially available in the US). Epinephrine also is absorbed following endotracheal administration, although serum concentrations achieved may be only 10% of those with an equivalent IV dose.. After oral inhalation of epinephrine in the usual dosage, absorption is slight and the effects of the drug are restricted mainly to the respiratory tract. Absorption increases somewhat when larger doses are inhaled, and systemic effects may occur. Metabolism / Metabolites Epinephrine is rapidly inactivated mainly by enzymic transformation to metanephrine or normetanephrine, either of which is then conjugated and excreted in the urine in the form of both sulfates and glucuronides. Either sequence results in the formation of 3-methoxy-4- hydroxy-mandelic acid(vanillylmandelic acid, VMA) which is shown to be detectable in the urine. Epinephrine is rapidly inactivated in the body mostly by the enzymes COMT (catechol-O-methyltransferase) and MAO (monoamine oxidase). The liver is abundant in the above enzymes, and is a primary, although not essential, tissue in the degradation process. The pharmacologic actions of epinephrine are terminated mainly by uptake and metabolism in sympathetic nerve endings. Circulating drug is metabolized in the liver and other tissues by a combination of reactions involving the enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). The major metabolites are metanephrine and 3-methoxy-4-hydroxymandelic acid (vanillylmandelic acid, VMA) both of which are inactive. About 40% of a parenteral dose of epinephrine is excreted in urine as metanephrine, 40% as VMA, 7% as 3-methoxy-4-hydroxyphenoglycol, 2% as 3,4-dihydroxymandelic acid, and the remainder as acetylated derivatives. These metabolites are excreted mostly as the sulfate conjugates and, to a lesser extent, the glucuronide conjugates. Only small amounts of the drug are excreted unchanged. Circulating epinephrine is metabolized in the liver and is taken up into adrenergic neurons and metabolized by MAO and catechol-O-methyltransferase to metadrenaline, sulfate conjugates, and hydroxy derivatives of mandelic acid. Epinephrine has known human metabolites that include Epinephrine sulfate. Biological Half-Life The plasma half-life is approximately 2-3 minutes. However, when administered by subcutaneous or intramuscular injection, local vasoconstriction may delay absorption so that epinephrine's effects may last longer than the half-life suggests. Elimination half life is 1 minute. Absorption: L-Adrenaline has poor oral bioavailability (~2-5% in humans) due to extensive first-pass metabolism by COMT and MAO. Subcutaneous absorption is rapid (peak plasma concentration at 15-30 minutes), and topical ocular absorption is minimal (~1-2% systemic) [1][2] Distribution: It distributes rapidly into tissues, with a volume of distribution (Vdss) of ~2-3 L/kg in humans. Brain penetration is limited by the blood-brain barrier [2][3] Metabolism: Primarily metabolized in the liver and tissues by COMT (to metanephrine) and MAO (to 3,4-dihydroxymandelic acid) [2][4] Excretion: The plasma elimination half-life is ~2-3 minutes in humans. Approximately 80-90% of the dose is excreted in urine as metabolites within 24 hours [2][4] Plasma protein binding: L-Adrenaline has a plasma protein binding rate of ~15-20% in humans [2][4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of epinephrine during breastfeeding. Because of its poor oral bioavailability and short half-life, any epinephrine in milk is unlikely to affect the infant. High intravenous doses of epinephrine might reduce milk production or milk letdown. Low-dose intramuscular (such as Epi-Pen), epidural, topical, inhaled or ophthalmic epinephrine are unlikely to interfere with breastfeeding. To substantially diminish the effect of the drug after using eye drops, place pressure over the tear duct by the corner of the eye for 1 minute or more, then remove the excess solution with an absorbent tissue. Epinephrine is the first line-medication of choice for treatment of anaphylaxis; it should be used in the same manner in breastfeeding and non-breastfeeding patients. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. Intravenous epinephrine infusion in nonnursing subjects and in women with hyperprolactinemia decreases serum prolactin concentrations. Animal data indicate that intraarterial epinephrine can decrease serum oxytocin and inhibit milk ejection. However, low-dose infusion of epinephrine as part of epidural analgesia does not impair breastfeeding in nursing mothers. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. An Egyptian study compared lidocaine 2% (n = 75) to lidocaine 2% plus epinephrine 1:200,000 (n = 70) as a wound infiltration following cesarean section. Patients who received epinephrine in combination with lidocaine began breastfeeding at 89 minutes following surgery compared to 132 minutes for those receiving lidocaine alone. The difference was statistically significant. Interactions Use in patients taking propranolol and other nonselective beta blockers may produce severe hypertension owing to blockade of beta-2-mediated vasodilation, resulting in unopposed alpha-vasoconstriction. Epinephrine should not be administered concomitantly with other sympathomimetic agents because of the possibility of additive effects and increased toxicity. Administration of epinephrine in patients receiving cyclopropane or halogenated hydrocarbon general anesthetics that increase cardiac irritability and seem to sensitize the myocardium to epinephrine may result in arrhythmias including PVCs, tachycardia, or fibrillation. Epinephrine is contraindicated for use with chloroform, trichloroethylene, or cyclopropane and should be used cautiously, if at all, with other halogenated hydrocarbon anesthetics such as halothane. Epinephrine may not be absorbed rapidly enough to cause serious adverse effects when applied topically as a hemostatic in patients undergoing short surgical procedures such as tonsillectomy and adenoidectomy using halothane anesthesia. Prophylactic administration of lidocaine or prophylactic IV administration of propranolol 0.05 mg/kg may protect against ventricular irritability if epinephrine is used during anesthesia with a halogenated hydrocarbon anesthetic. In one study, arrhythmias occurring after parenteral use of epinephrine during general anesthesia responded promptly to IV propranolol 0.05 mg/kg. The effects of epinephrine 1/200,000 added to mg of epidural morphine were investigated in 3 healthy male volunteers, during 26 hr observation sessions. Cutaneous hypalgesia was intense, faster in onset, and longer in duration after epinephrine-morphine than after plain morphine. Apparently, epinephrine 1/200,000 reduces manifestations of cord and brainstem uptake. The need for the reduction of the customary dose of epidural morphine, while epinephrine is used as an adjuvant, is discussed. For more Interactions (Complete) data for EPINEPHRINE (20 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat dermal 62 mg/kg LD50 Rat sc 62 mg/kg LD50 Rat iv 0.15 mg /kg LD50 Rat im 3500 mg/kg For more Non-Human Toxicity Values (Complete) data for EPINEPHRINE (9 total), please visit the HSDB record page. Common adverse effects in humans include palpitations (incidence ~30%), tachycardia (~25%), hypertension (~18%), and tremor (~12%), which are dose-related and reversible [2][4] Acute intravenous LD50 in mice is ~9 mg/kg; lethal doses induce severe ventricular arrhythmias, myocardial ischemia, and convulsions [2][4] Topical ocular administration may cause eye irritation (~8%) and transient mydriasis (~5%) in humans [1] |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Adrenergic alpha-Agonists; Adrenergic beta-Agonists; Adrenergic Agonists; Bronchodilator Agents; Mydriatics; Sympathomimetics; Vasoconstrictor Agents Epinephrine is the drug of choice in the emergency treatment of severe acute anaphylactic reactions including anaphylactic shock. Symptoms such as urticaria, pruritus, angioedema, and swelling of the lips, eyelids, and tongue which may result from reactions to drugs, sera, insect stings, food, or other allergens may be relieved by epinephrine. Epinephrine should be given to all patients with signs of systemic reactions, particularly hypotension, airway swelling, or definite breathing difficulty. Circulatory support during anaphylactic shock requires rapid volume resuscitation and vasopressor therapy to support blood pressure; epinephrine is the drug of choice for the treatment of both vasodilation/hypotension and cardiac arrest associated with anaphylaxis. /Included in US product label/ Epinephrine may be added to solutions of some local anesthetics to decrease the rate of vascular absorption of the anesthetic, thereby localizing anesthesia and prolonging the duration of anesthesia; the risk of systemic toxicity from the local anesthetic is also decreased. Epinephrine may be applied topically to control superficial bleeding from arterioles or capillaries in the skin, mucous membranes, or other tissues. Bleeding from larger vessels is not controllable by topical application of epinephrine. /Included in US product label/ Epinephrine is used for its a-adrenergic stimulatory effects to increase blood flow in advanced cardiovascular life support (ACLS) during cardiopulmonary resuscitation (CPR). The principal beneficial effects of the drug in patients with cardiac arrest result from increases in aortic diastolic blood pressure and in myocardial and cerebral blood flow during resuscitation. The value and safety of the beta-adrenergic effects of epinephrine are controversial because they may increase myocardial work and reduce subendocardial perfusion. Epinephrine remains a drug of choice and a high priority for ACLS in cardiac arrest to facilitate return of spontaneous circulation. /Included in US product label/ For more Therapeutic Uses (Complete) data for EPINEPHRINE (15 total), please visit the HSDB record page. Drug Warnings Epinephrine should not be used in cardiogenic shock because it increases myocardial oxygen demand, nor should it be used in hemorrhagic or traumatic shock. Vet: epinephrine injection (1:1000): do not use in acute hypotension produced by phenothiazine derived tranquilizers, since further depression of blood pressure can occur. Do not use when cyclopropane or halogenated anesthetics are used because of possible cardiac collapse. Do not use in treatment of vascular shock. Do not use in patients known to be sensitive to epinephrine ... Use with caution in hyperthyroid animals; animals being treated with thyroid, digitalis, or mercurial diuretics. Do not use injection if it is brown or contains a precipitate. A prospective study where topical epinephrine was used on burn and non-burn patients and five patients served as controls without epinephrine usage. Catecholamine concentrations were measured and to estimate the systemic effects of epinephrine, serum lactate and pyruvate concentrations were analyzed and perioperative haemodynamic changes recorded. Compared to the baseline values, there was a significant increase in the heart rate, serum epinephrine and lactate concentrations and LP-ratios in the burn patients and an increase in the epinephrine concentrations in the non-burn patients at 1 and 2 h. Epinephrine and lactate concentrations and LP-ratios were also higher in the burn patients compared to the other groups. Altogether, there were no changes in the control group. This study showed that the use of topical epinephrine has systemic effects on hemodynamics and serum epinephrine concentrations. Increased epinephrine concentrations in burn patients suggest increased absorption properties in these patients. The increased lactate concentrations and LP-ratios suggest tissue ischaemia, likely in skin. Some manufacturers state that epinephrine is contraindicated for parenteral use during the second stage of labor; parenteral administration of the drug to maintain blood pressure during spinal anesthesia for delivery can cause acceleration of fetal heart rate and should not be used in obstetric patients when maternal systolic/diastolic blood pressure exceeds 130/80 mm Hg. Epinephrine should be administered cautiously by oral inhalation to pregnant patients. Epinephrine should be used during pregnancy only if the potential benefits justify the possible risks to the fetus. There is some evidence that epidural administration of lidocaine with epinephrine during labor is safe. For more Drug Warnings (Complete) data for EPINEPHRINE (21 total), please visit the HSDB record page. Pharmacodynamics Epinephrine is a sympathomimetic drug. It causes an adrenergic receptive mechanism on effector cells and mimics all actions of the sympathetic nervous system except those on the facial arteries and sweat glands. Important effects of epinephrine include increased heart rate, myocardial contractility, and renin release via beta-1 receptors. Beta-2 effects produce bronchodilation which may be useful as an adjunct treatment of asthma exacerbations as well as vasodilation, tocolysis, and increased aqueous humor production. In croup, nebulized epinephrine is associated with both clinically and statistically significant transient reduction of croup symptoms 30 minutes post-treatment. Epinephrine also alleviates pruritus, urticaria, and angioedema and may be helpful in relieving gastrointestinal and genitourinary symptoms associated with anaphylaxis because of its relaxing effects on the smooth muscle of the stomach, intestine, uterus, and urinary bladder. L-Adrenaline is the naturally occurring enantiomer of adrenaline, a non-selective adrenoceptor agonist [1][2][3][4] Its mechanism involves activating α-adrenoceptors (vasoconstriction, increased blood pressure, reduced ocular blood flow) and β-adrenoceptors (cardiac stimulation, bronchial relaxation, enhanced synaptic plasticity) [1][2][3][4] Clinically indicated for the emergency treatment of anaphylaxis, cardiac arrest, and severe bronchospasm; topical ocular formulations are used to reduce intraocular pressure and control bleeding during eye surgery [1][2][4] It enhances age-related memory impairment via CREB phosphorylation in the hippocampus, suggesting potential neuroprotective roles [3] Due to rapid metabolism and short half-life, it is administered via subcutaneous, intravenous, or topical routes (oral use is not recommended) [2][4] |
| 分子式 |
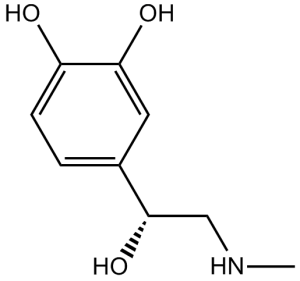
C9H13NO3
|
|
|---|---|---|
| 分子量 |
183.2
|
|
| 精确质量 |
183.089
|
|
| 元素分析 |
C, 59.00; H, 7.15; N, 7.65; O, 26.20
|
|
| CAS号 |
51-43-4
|
|
| 相关CAS号 |
L-Epinephrine sulfate; 52455-32-0
|
|
| PubChem CID |
5816
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
413.1±40.0 °C at 760 mmHg
|
|
| 熔点 |
208-211ºC
|
|
| 闪点 |
207.9±17.9 °C
|
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
|
| 折射率 |
1.608
|
|
| LogP |
-0.63
|
|
| tPSA |
72.72
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
13
|
|
| 分子复杂度/Complexity |
154
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
O[C@H](C1=CC(O)=C(O)C=C1)CNC
|
|
| InChi Key |
UCTWMZQNUQWSLP-VIFPVBQESA-N
|
|
| InChi Code |
InChI=1S/C9H13NO3/c1-10-5-9(13)6-2-3-7(11)8(12)4-6/h2-4,9-13H,5H2,1H3/t9-/m0/s1
|
|
| 化学名 |
4-[(1R)-1-hydroxy-2-(methylamino)ethyl]benzene-1,2-diol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 (3). 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.4585 mL | 27.2926 mL | 54.5852 mL | |
| 5 mM | 1.0917 mL | 5.4585 mL | 10.9170 mL | |
| 10 mM | 0.5459 mL | 2.7293 mL | 5.4585 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Intrathecal Dexmedetomidine Vs Epinephrine
CTID: NCT06418308
Phase: Phase 4 Status: Recruiting
Date: 2024-10-15