| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
Somatostatin receptors
|
|---|---|
| 体外研究 (In Vitro) |
BIM 23014,Lanreotide/兰瑞肽,100 nM; 0-48小时)增加了辐射引起的细胞凋亡[1]。服用兰瑞肽后,GH3 细胞集落形成单位以剂量依赖性方式减少。兰瑞肽剂量为 1、10、100 和 1000 nM 时,细胞存活率分别为 75%、56%、39% 和 27%。 IC50[1] 为 57 nM。在体外,lenreotide 抑制分泌生长激素的垂体腺瘤细胞的生长和激素释放[2]。
GH3细胞对Lanreotide和γ辐射的剂量反应[1] 如图1A所示,用Lanreotide处理导致GH3细胞集落形成单位的剂量依赖性减少。Lanreotide的剂量分别为1、10、100和1000 nM的细胞存活率分别为75%、56%、39%和27%。IC50(50%抑制细胞生长)为57 nM.辐射存活曲线如图1B所示。GH3细胞具有典型的辐射剂量-反应存活曲线,在剂量低于5 Gy和高剂量下的直线。SF在2 Gy(SF2,每日分次放疗中常用的剂量)为40%。 兰瑞肽对GH3细胞辐射反应的影响[1] 将GH3细胞在组织培养皿中放置过夜。辐射存活曲线如图2所示。单独使用兰瑞肽治疗,剂量为100或1000 nM为48 与未经治疗的对照组相比,没有辐射的h使克隆存活率降低了5-10%。不含兰瑞肽的单独辐射产生了剂量依赖性生存曲线,SF2为48-55%。用100剂量的兰瑞肽治疗 nM为48 h之前(48小时前和24小时前) h前拉瑞肽)或辐射时(0 h前拉瑞肽)产生的存活曲线略有向下偏移,并在7-10的剂量下分离 从单独辐射而不使用兰瑞肽产生的存活曲线中得出的Gy(图2A)表明,兰瑞肽增强了GH3细胞的辐射反应。SF 10 单独辐射的Gy分别为0.0006、0.00022、0.00040和0.00042,48 h前,24 h前和0 h预先接触兰瑞肽(图2C)。然而,治疗1000 nM兰瑞肽没有改变辐射存活曲线的形状和斜率,表明在这些实验条件下没有辐射防护(或辐射增敏)作用(图2B)。 兰瑞肽和辐射对细胞凋亡和细胞周期分布的影响[1] 48岁时处于亚G1期、G1期、S期和G2/M期的细胞百分比 h分别为1.4±0.2、73.2±1.0、8.4±1.0和16.9±1.8%。治疗100 单独使用nMLanreotide导致亚G1、G1、S和G2/M期分布分别为2.28±0.3、73.8±1.1、7.72±0.8和16.2±0.5%,表明与未处理的对照组相比,使用Lanreotide治疗对细胞周期特征没有显著影响,除了凋亡的亚G1细胞从1.4%适度增加到2.28%。治疗10 Gy辐射导致G1期细胞比例从73.2%下降到48 h.与此同时,G2/M期细胞从辐射前的16.9%增加到48℃时的35.7% 照射后h,细胞在G2/M期停滞长达168 h不得释放。亚二倍体细胞群代表凋亡细胞,在辐射后从基线1.4%稳步增加到168℃时的峰值约12% h(图3)。用辐射和兰瑞肽联合治疗GH3细胞产生了与没有兰瑞肽的受照射细胞相似的细胞周期特征,除了凋亡亚G1比例的增加。如图3所示,在48 照射后h,凋亡的亚G1细胞从单独照射的4.9%增加到照射与48、24和0的组合的8.6、9.3和13.4% h分别暴露于兰瑞肽前,与单独辐射相比增加了77-173%(P<0.01)。168 放疗后h,单独放疗的亚G1细胞分数为12%,放疗加兰瑞肽的亚G1淋巴细胞分数为20-22%,增加了67-83%(P<0.01)。 所有分析的腺瘤均表达至少一种生长抑素受体亚型mRNA。77%的腺瘤中鉴定出SSTR2 mRNA,69%的腺瘤中检测到SSTR1和SSTR3,60%的腺瘤中识别出SSTR5。生长抑素和兰瑞肽抑制佛波酯(PMA)刺激条件下的细胞增殖(10/13个腺瘤),以及胎牛血清(3/3个瘤)或IGF-I刺激(2/2个腺瘤)后的细胞增殖。相反,在生长抑素存在或不存在的情况下,GHRH或毛喉素治疗对腺瘤细胞的DNA合成没有显著影响(分别为2/2和4/4腺瘤)。钒酸盐预处理逆转了生长抑素对PMA诱导的DNA合成的抑制作用,表明酪氨酸磷酸酶参与了这种作用(2/2腺瘤);生长抑素治疗后,两个腺瘤中酪氨酸磷酸酶活性的直接诱导证实了这一点。生长抑素和兰瑞肽显著抑制了佛波酯、毛喉素、GHRH和KCl依赖性的培养基中GH分泌的增加。此外,在微荧光分析中,40mm KCl去极化诱导的电压敏感钙通道活性显著降低(5/5腺瘤)。 结论:这些数据表明,生长抑素和Lanreotide在体外抑制人GH分泌垂体腺瘤细胞的增殖和激素释放,并表明酪氨酸磷酸酶的激活可能代表介导抗增殖作用的细胞内信号,抑制电压依赖性钙通道和腺苷酸环化酶活性可能控制GH分泌[2]。 |
| 体内研究 (In Vivo) |
给予兰瑞肽/Lanreotide(2.5–10 mg/kg;皮下注射;每天一次,持续 5 天)可抑制肿瘤生长[1]。
GH3肿瘤对Lanreotide的剂量反应[1] 将已建立GH3异种移植物肿瘤的裸鼠组皮下注射2.5、5、10、20或50 mg/kg兰瑞肽每日一次,持续5天。剂量基于先前利用兰瑞肽体内给药的研究(Prevost等人,1994年;Melen-Mucha等人,2004年)。如图4所示,兰瑞肽对GH3肿瘤生长具有钟形剂量依赖性作用,最佳剂量范围很窄。最大的肿瘤生长抑制作用(即最长的TGD时间13.1±4.7天)发生在每日10 当每日兰瑞肽剂量较高时(即20和50 mg/kg)或更低(2.5和5 mg/kg)大于10 lanreotide对GH3肿瘤生长的影响减弱。 在这些研究中,与未经治疗的对照组小鼠相比,所有测试剂量的Lanreotide均未导致体重显著下降(数据未显示)。此外,用兰瑞肽治疗的荷瘤小鼠的总体外观和日常活动没有明显变化。 Lanreotide每日一次与每日两次的剂量方案比较[1] 为了确定兰瑞肽剂量方案对肿瘤生长的重要性,我们比较了每日单次剂量(qd)和每日两次剂量(bid)后的肿瘤大小。携带GH3肿瘤的裸鼠皮下注射兰瑞肽,剂量为2.5、5或10 mg/kg,连续5天,每天一次或每天两次(间隔8小时)。如图5所示,每天一次或每天两次以相同剂量治疗的组之间没有统计学上的显著差异(P=0.3-0.9)。然而,Lanreotide在10 在所有研究的剂量方案中,mg/kg每日一次产生的TGD时间最长(4.9±2.1天)(P<0.05)。值得注意的是,这比5天后的(1.1±3.1天)要长 mg/kg,每日两次。类似地,单次每日剂量为5 mg/kg qd产生的TGD时间比2.5 mg/kg bid。这些数据表明,在相同的总日剂量下,单剂量兰瑞肽产生的肿瘤生长抑制作用至少与分次给药方案一样多。因此,进一步的研究采用了单次每日给药方案。 兰瑞肽与分次放疗的联合治疗[1] 为了研究兰瑞肽对放射治疗肿瘤反应的影响,用以下药物治疗已建立GH3肿瘤的裸鼠:1)10 每日mg/kg兰瑞肽,持续5天;2)连续5天每天局部肿瘤放疗,剂量为250、200或150 cGy/每日分数;3)兰瑞肽和上述局部肿瘤放疗的组合;或4)作为未经治疗的对照,每天皮下注射生理盐水(0.005ml/g体重)。在联合治疗中,注射兰瑞肽20 辐射前分钟。数据如图6所示(肿瘤生长曲线)。单独服用Lanreotide,剂量为10 mg/kg适度抑制GH3肿瘤的生长,4×TGD时间为4.5至8.3天(与相关对照组相比,P=0.3-0.06)。单独分次局部肿瘤放疗可显著抑制肿瘤生长,并在250天内产生35.1±5.7天的TGD时间 cGy分数,21.7±5.5 200天 cGy分数为16.7±1.7天,持续150天 cGy组分。兰瑞肽与250、200或150辐射的组合 cGy/fraction持续5天抑制肿瘤生长,产生的TGD时间与单独辐射相似(P>0.05)。此外,与单独的分次放射治疗相比,兰瑞肽和分次放射的联合治疗不会导致动物体重进一步下降。 Lanreotide联合放射治疗的预给药[1] 为了研究预给药兰瑞肽是否可以调节辐射对肿瘤生长的影响,用以下药物治疗患有GH3异种移植物肿瘤的裸鼠:1)10 每日mg/kg兰瑞肽,持续10天; 2) 150 连续5天每天进行cGy局部肿瘤放疗;3)10 mg/kg兰瑞肽5天,然后联合服用兰瑞肽和150 连续5天每天进行cGy辐射。一组携带肿瘤的小鼠每天皮下注射生理盐水10天,也作为未经治疗的对照。如图7所示,剂量为10 10天的mg/kg剂量适度抑制了肿瘤生长(4×TGD,8.3±8.3天,与对照组相比P=0.06)。局部肿瘤放疗150 cGy抑制肿瘤生长,TGD时间为15.5.0±8.8天(与对照组和单独使用兰瑞肽相比P<0.05)。在该治疗方案中,兰瑞肽预给药和放疗的联合治疗导致TGD时间为15.1±8.6天,与单独放疗产生的TGD时间(15.5±8.8天;P>0.05)相似。在单独放射治疗组和联合治疗组中,有两只小鼠的肿瘤完全消退,60天后研究终止时没有肿瘤再生。此外,就体重减轻、一般外观、皮肤反应或小鼠活动水平而言,单独使用兰瑞肽和与辐射联合使用不会产生任何明显的全身毒性迹象(数据未显示)。 |
| 细胞实验 |
细胞凋亡分析[1]
细胞类型: GH3 测试浓度: 100 nM 孵育时间: 48 小时, 24 小时或辐射前立即(0 小时) 实验结果:与单独辐射相比,凋亡亚 G1 比例增加。 体外克隆形成试验[1] GH3细胞对Lanreotide和辐射治疗的剂量反应通过克隆形成试验进行了表征。用0.05%胰蛋白酶-EDTA溶液分离GH3细胞,计数并在60 mm培养皿,稀释度为100-100 000 新鲜生长培养基中的细胞/培养皿一式三份。将Lanreotide 以0-1000的最终浓度添加到平板中 nM.细胞用0-10 室温下使用剂量率为300的137Cs源的Gy cGy/min。暴露于兰瑞肽或γ射线后,取出培养基,用PBS溶液洗涤培养皿两次,并装满新鲜的生长培养基。37℃孵育后 在°C下放置21天,细胞用0.25%结晶紫染色。在解剖显微镜下计数含有≥50个细胞的菌落,并绘制存活曲线。镀覆效率(PE)计算为生长成集落的镀覆细胞的百分比。存活分数(SF)定义为干预后存活的细胞分数,即菌落数/(菌落数×PE)。[1] 对于辐照实验,最终浓度为100或1000 nM的Lanreotide (通过图1所示的实验确定)在48、24或0时加入 辐射前h。细胞用0-10 室温下使用Cs-137γ辐照器在兰瑞肽存在下进行Gy辐照。辐射后,将预先暴露于兰瑞肽24小时或0小时的细胞在含兰瑞肽的培养基中再孵育24或48小时 h分别。在总共48小时的暴露后,去除含兰瑞肽的培养基,用PBS溶液洗涤培养皿两次,然后填充新鲜的生长培养基。在没有接触兰瑞肽的情况下照射的盘子也用PBS清洗了两次,并重新装满了新鲜的培养基。将细胞孵育21天以形成集落。 细胞凋亡和细胞周期分析[1] 将GH3细胞置于60 mm培养皿(50万个细胞/皿)培养过夜。Lanreotide在100 在48℃时加入nM h, 24 h、 或在辐射前立即(0小时)。用10 室温下的Gyγ辐射。收集48、72、96和168个细胞 照射后h,用冷PBS加5 mM EDTA。将细胞重新悬浮在冷的PBS-EDTA溶液中,并用冷的100%乙醇固定。孵育30天后 在室温下,细胞被造粒,并用100 μg/ml RNase A在PBS-EDTA溶液中30分钟 在室温下保持min。加入碘化丙啶(PI)至终浓度为50 μg/ml。用FACScan流式细胞仪分析DNA含量。计算亚G1期(凋亡)、G1期、S期和G2/M期细胞的百分比。未经任何处理的对照细胞在168天内显示出一致的细胞周期分布 h(数据未显示)。 细胞治疗[2] GH分泌腺瘤细胞以1×105的密度铺在24孔板上。24小时后,细胞被血清和生长因子饥饿24小时。随后,细胞用受试物质处理16小时。在孵育时间结束时,在某些情况下,当有少量细胞可用时,取出一等分培养基进行GH测定,然后在增殖试验前加入2µCi/ml的[3H]-胸苷4小时。然而,在可能的情况下,对细胞增殖和GH释放进行了独立治疗。 增殖试验[2] 如前所述(Florio等人,1992),通过[3H]-胸苷摄取试验测量DNA合成活性。简而言之,按照指示处理的细胞被胰蛋白酶消化(在37°C下15分钟),在10%三氯乙酸(TCA)中提取,并在真空下通过玻璃纤维过滤器过滤。然后在真空下用10%和5%TCA和95%乙醇依次洗涤过滤器。然后在闪烁计数器中计数TCA不溶部分。 体外生长激素释放测量[2] 如前所述,GH是通过商业免疫放射分析法测量的(Giusti等人,2002)。将细胞培养基在磷酸盐缓冲盐水(PBS)缓冲液中从1:10稀释到1:100,以更好地评估GH含量。来自同一实验的所有样品都在同一测定中进行了测量。结果以µg/孔表示。对照数据的绝对和百分比变化以平均值±SEM的形式报告。 PTP测定[2] 细胞在6-cm培养皿中以50%的融合率铺板,在37°C的无FCS培养基中在CO2培养箱中与受试物质预孵育1小时。然后用PBS洗涤细胞,在含有0.32 m蔗糖、10 mm Tris、pH 7.5、5 mm EGTA、1 mmol/l EDTA的缓冲液中机械刮擦,并超声处理。然后将细胞裂解液在4°C下以15000 g离心60分钟,重新悬浮在含有250 mm HEPES、pH 7.2、140 mm NaCl、1%NP40和苯甲基磺酰氟(PMSF)以及亮肽作为蛋白酶抑制剂的缓冲液中,并使用Bradford(1976)的方法以牛血清白蛋白为标准和Bio-Rad试剂测定蛋白质含量。PTP测定中使用了20微克对照或处理过的膜。在分光光度测定中,使用合成底物对硝基苯基磷酸酯(p-Npp)进行PTP测定。p-Npp是一种通用的磷酸酶底物,在Ser/Thr磷酸酶抑制剂存在的情况下,它对PTP具有特异性(Pan等人,1992)。将膜在30°C下在80µl体积中预孵育5分钟,其中含有20µl 5×反应缓冲液[250 mm HEPES,pH 7.2,50 mm二硫苏糖醇(DTT),25 mm EDTA和500 nm微囊藻毒素-亮氨酸-精氨酸,以色列耶路撒冷阿拉莫实验室,作为Ser/Thr磷酸酶抑制剂]。通过加入20µl 50 mmp Npp开始反应,在30°C下进行30分钟,并通过加入900µl 0.2 N NaOH停止反应。在410nm处测量样品的吸光度,该吸光度与去磷酸化底物的量成正比。在该波长下,p-Npp的消光系数为1.78×104m/cm。 单细胞水平细胞内钙浓度的测量[2] 将细胞铺在25毫米干净的玻璃盖玻片上,之前涂有聚赖氨酸(10µg/ml),然后转移到35毫米的培养皿中。24-48小时后,将细胞血清饥饿24小时。在实验当天,用平衡盐溶液(HEPES 10 mm,pH 7.4;NaCl 150 mm;KCl 5.5 mm;CaCl2 1.5 mm;MgSO4 1.2 mm;葡萄糖10 mm)洗涤细胞10分钟。然后在室温下用Fura-2五乙酰氧基甲酯(4µm)装载腺瘤细胞60分钟。荧光测量如先前报道的那样进行(Florio等人,1999b)。简而言之,将盖玻片安装在盖玻片室中,使用尼康40X/1·3 NA Fluor DL物镜,用倒置尼康膜片显微镜对fura-2荧光进行成像。用带石英聚光透镜的氙灯照射细胞。快门和包含两种不同激发滤光片(340nm和380nm)的滤光轮由计算机控制。发射的光穿过400nm二向色镜,在490nm处过滤,并由与光增强器连接的CCD相机收集。图像在连接到配备Quanticell软件 的计算机的图像处理器中数字化并求平均值。为了校准荧光信号,我们使用了负载Fura-2的细胞;Rmax和Rmin分别是饱和和零[Ca2+]i时的比值,是通过用含有CaCl2(10 mm)、洋地黄皂苷(2.5µm)和离子霉素(2µm)的盐溶液灌注细胞,然后用含有EGTA(10毫米)的无Ca2+盐溶液灌注而获得的。根据Grynkiewicz等人(1985)的方程,使用Quanticell软件,将获得的Rmax和Rmin值表示为灰度平均值,用于计算细胞内Ca2+浓度([Ca2+]i)。 |
| 动物实验 |
Animal/Disease Models: Male nude mice, 8 weeks old and 20–25 g in body weight (GH3 tumor-bearing nude mice) [1]
Doses: 2.5, 5, 10 mg/kg Route of Administration: Subcutaneous; daily for 5 days Experimental Results: Produced tumor growth inhibition. Mouse xenograft tumor model and therapy [1] Male nude mice, 8 weeks old and 20–25 g in body weight were tested and found to be negative for specific pathogens. The mice were normally bred and maintained under specific pathogen-free conditions, and sterilized food and water were available ad libitum. Mice were injected subcutaneously in the right flank with 5×106 tumor cells in 100 μl of a Hank's solution and Matrigel 1:1 mixture. One tumor per mouse was inoculated. When tumors reached an average size of 120 mm3 (80–200 mm3), mice were randomly assigned to the different treatment groups. Five to eight mice were used in each group. Lanreotide was injected s.c. at doses specified in each experiment. For radiation, the unanesthetized tumor-bearing mice were placed in individual lead boxes with tumors protruding through a cutout window at the rear of each box. The radiation was delivered using a Philips RT-250 200 kVp X-ray unit (12.5 mA; half value Layer, 1.0-mm Cu) at a dose rate of 138 cGy/min. Tumors were locally irradiated with a dose of 150–250 cGy per fraction daily for 5 consecutive days as specified in each experiment. The length and width of the tumors were measured with calipers before treatment by the same investigator, and three times a week thereafter until the tumor volume reached at least four times (4×) the pretreatment volume. The tumor volume was calculated using the formula: tumor volume (mm3)=π/6×length×width2. The tumor volume quadrupling (4×) time was determined by a best-fit regression analysis. The tumor growth delay (TGD) time (in days) is the difference between the tumor volume quadrupling time of treated tumors compared with that of untreated control tumors. Both the tumor volume quadrupling time and TGD time were calculated for each individual animal and then averaged for each group. In some experiments, a complete regression of tumors was recorded if a tumor completely shrunk to the point that it was not palpable at the end of the experiment. Body weight was measured twice a week. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Lanreotide forms a drug depot at the site of injection; therefore, there are 2 phases that describe the absorption of Lanreotide: 1. Initial rapid subcutaneous release during the first few days of treatment where drug that has not precipitated is rapidly absorbed. 2. Slow release of drug from the depot via passive diffusion. Absorption is independent of body weight, gender, and dosage. <5% of lanreotide is excreted in urine, and less than 0.5% is excreted unchanged in the feces suggesting biliary excretion involvement. Estimated Volume of Distribution = 15.1 L Estimated Clearance = 23.1 L/h Biological Half-Life Half-life is approximately 22 days |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration studies of lanreotide, serum enzyme levels did not change appreciably and there were no reports of clinically apparent acute liver injury. Pooled analyses reported that there were no overall changes in serum ALT, AST or alkaline phosphatase levels during therapy or instances of clinically meaningful elevations with treatment. Prolonged therapy with lanreotide, as with other somatostatin analogues, was associated with a high rate of biliary sludge and cholelithiasis, probably due to inhibition of gall bladder contractility and decrease in bile secretion. In long term studies, cholelithiasis developed in 20% to 33% of lanreotide treated patients. In some instances, symptomatic cholecystitis occurred which can be accompanied by mild-to-moderate elevations in serum enzymes and bilirubin. However, most lanreotide associated gallstones were asymptomatic. Unlike octreotide, lanreotide and other long acting somatostatin analogues have not been liked to cases of clinically apparent liver injury, independent of cholelithiasis or biliary sludge, although they have had more limited use and have not been used in many of the clinical situations that were treated with octreotide (portal hypertension, variceal hemorrhage and infants with congenital hyperinsulinemia). Likelihood score: E* (unproven but suspected rare cause of clinically apparent hepatobiliary injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation The excretion of lanreotide into breastmilk has not been studied. However, because it has a high molecular weight of 1096 daltons it is likely to be poorly excreted into breastmilk and it is a peptide that is likely digested in the infant's gastrointestinal tract, so it is unlikely to reach the clinically important levels in infant serum. Lanreotide has been given by injection to newborn infants with congenital hyperinsulinemia; reversible mild elevation of liver enzymes occurred in some infants. The manufacturer states that women should not breastfeed during treatment with depot lanreotide and for 6 months following the last dose. ◉ Effects in Breastfed Infants A woman with acromegaly was treated with lanreotide Autogel 120 mg monthly, cabergoline 2 mg weekly and pegvisomant 80 mg weekly. She breastfed (extent not stated) her infant and they were followed for 12 years. Her child had normal growth and development. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
Lanreotide is a drug employed in the management of acromegaly (a hormonal condition caused by excess growth hormone) in addition to symptoms caused by neuroendocrine tumors, especially carcinoid syndrome. This drug is a long-acting analog of the drug somatostatin, a growth hormone inhibitor. Lanreotide is manufactured by the company, Ipsen Pharmaceuticals as lanreotide acetate, and marketed as Somatuline. It is approved in several countries worldwide, including the United Kingdom, Australia, and Canada. Lanreotide was first approved for use in the United States by the FDA on August 30, 2007.
Lanreotide is a synthetic polypeptide analogue of somatostatin that resembles the native hormone in its ability to suppress levels and activity of growth hormone, insulin, glucagon and many other gastrointestinal peptides. Because its half-life is longer than somatostatin, lanreotide can be used clinically to treat neuroendocrine tumors that secrete excessive amounts of growth hormone (acromegaly) or other active hormones or neuropeptides. Lanreotide has many side effects including suppression of gall bladder contractility and bile production, and maintenance therapy may cause cholelithiasis and pancreatitis as well accompanying liver injury. Lanreotide is a synthetic cyclic octapeptide analogue of somatostatin. Lanreotide binds to somatostatin receptors (SSTR), specifically SSTR-2 and also to SSTR-5 with a lesser affinity. However, compare with octreotide, this agent is less potent in inhibiting the release of growth hormone from the pituitary gland. Furthermore, lanreotide has an acute effect on decreasing circulating total and free insulin-like growth factor 1 (IGF-I). This agent is usually given as a prolonged-release microparticle or Autogel formulation for the treatment of acromegaly and to relieve the symptoms of neuroendocrine tumors. Lanreotide Acetate is the acetate salt of a synthetic cyclic octapeptide analogue of somatostatin. Lanreotide binds to somatostatin receptors (SSTR), specifically SSTR-2 and also to SSTR-5 with a lesser affinity. However, compare with octreotide, this agent is less potent in inhibiting the release of growth hormone from the pituitary gland. Furthermore, lanreotide has an acute effect on decreasing circulating total and free insulin-like growth factor 1 (IGF-I). This agent is usually given as a prolonged-release microparticle or Autogel formulation for the treatment of acromegaly and to relieve the symptoms of neuroendocrine tumors. See also: Lanreotide Acetate (has salt form). Drug Indication Lanreotide is indicated for the long-term treatment of patients with acromegaly who have had an inadequate response to, or cannot be treated with, surgery and/or radiotherapy. It is also indicated in the treatment of adult patients with unresectable, well- or moderately-differentiated, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs) to improve progression-free survival. Lanreotide is additionally indicated for the treatment of adults with carcinoid syndrome - when used, it reduces the frequency of short-acting somatostatin analog rescue therapy. FDA Label Treatment of acromegaly, Treatment of gastrointestinal fistulae, Treatment of metastases to peritoneum, Treatment of pituitary gigantism, Treatment of pituitary neoplasms Mechanism of Action Lanreotide is a somatostatin analogue (SSA) and has mainly inhibitory effects which are mediated via somatostatin receptors (SSTRs) 2 and 5 and include inhibition of growth hormone release in the brain. Tumor SSTR activation induces downstream cell cycle arrest and/or apoptosis, and also results in blunted production of substances that support tumor growth as well as tumor angiogenesis. This leads to the anti-proliferative effects of Lanreotide. Somatostatin analogs are a mainstay of medical therapy in patients with GH producing human pituitary tumors, and it has been suggested that somatostatin analogs may be radioprotective. We utilized GH secreting rat GH3 cells to investigate whether a somatostatin analog may limit the effects of radiation on proliferation and apoptosis in vitro and on tumor growth in vivo. Treatment with lanreotide alone at doses of either 100 or 1000 nM for 48 h reduced clonogenic survival by 5-10%. Radiation alone produced a dose-dependent survival curve with a SF2 of 48-55%, and lanreotide had no effect on this curve. The addition of lanreotide resulted in a 23% increase in the proportion of apoptotic sub-G1 cells following irradiation (P<0.01). In a mouse GH3 tumor xenograft model, lanreotide 10 mg/kg moderately inhibited the growth of GH3 tumors, with a 4x tumor growth delay (TGD) time that ranged from 4.5 to 8.3 days. Fractionated local tumor radiation alone significantly inhibited tumor growth and produced a TGD of 35.1+/-5.7 days for 250 cGy fractions. The combination of lanreotide, either antecedent to or concurrent, with radiation of 250, 200 or 150 cGy/fraction for 5 days inhibited tumor growth and produced the TGD times that were similar to radiation alone (P>0.05). Pretreatment with lanreotide had the most significant radiosensitizing effect. These studies demonstrate that the somatostatin analog lanreotide is not radioprotective in GH3 cells, and further studies are necessary to determine the impact of lanreotide on apoptosis.[1] In summary, using a mouse xenograft model, we have demonstrated that the somatostatin analog lanreotide does not protect pituitary tumor cells from the effects of ionizing radiation, but does promote radiation induced apoptosis in rat GH3 cells. Further studies, both in vitro and in vivo, are needed to determine the potential clinical relevance of these findings to the management of patients with neuroendocrine tumors, such as acromegaly, and whether somatostatin analogs should be administered concurrently with radiation therapy. These preliminary findings also suggest that lanreotide may enhance radiation-induced apoptosis and warrant further study. In addition, further studies are necessary to elucidate the mechanisms underlying this potential synergistic action of lanreotide and radiation on apoptosis induction.[1] Objective: Somatostatin is an endogenous inhibitor of hormone secretion and cell proliferation. Treatment with somatostatin analogues in humans causes a reduction in size and secretory activity of endocrine tumours, including GH-secreting pituitary adenomas. This study was aimed to characterize the intracellular mechanisms mediating the in vitro antiproliferative and antisecretory effects of somatostatin and its analogue lanreotide, on primary cultures of GH-secreting pituitary adenoma cells. Design: Thirteen GH-secreting pituitary adenoma postsurgical specimens were analysed for somatostatin receptor (SSTR) mRNA expression and a subset of them was analysed in vitro for the effect of somatostatin on cell proliferation, assessed by means of [3H]-thymidine uptake, and GH release, using an immunoradiometric assay. Moreover, the intracellular signalling involved in such effects has been studied.[2] In conclusion, our data show that, in GH-secreting adenomatous cells, the in vitro activation of SSTR by somatostatin or Lan causes a reduction in both DNA synthesis and GH secretion. This effect is correlated to an increase in phosphotyrosine phosphatase PTP activity for the antiproliferative effects, and an inhibition of voltage-dependent calcium channel activity for the antihormonal effects, that represent possible intracellular effectors of SSTR activation in pituitary adenoma cells.[2] |
| 分子式 |
C54H69N11O10S2
|
|---|---|
| 分子量 |
1096.32336
|
| 精确质量 |
1095.467
|
| 元素分析 |
C, 59.16; H, 6.34; N, 14.05; O, 14.59; S, 5.85
|
| CAS号 |
108736-35-2
|
| 相关CAS号 |
Lanreotide acetate;2378114-72-6;Lanreotide diTFA;1024499-83-9
|
| PubChem CID |
6918011
|
| 序列 |
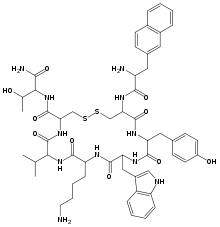
H-D-2Nal-Cys(1)-Tyr-D-Trp-Lys-Val-Cys(1)-Thr-NH2; 3-(2-naphthyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-threoninamide (2->7)-disulfide
|
| 短序列 |
XCYWKVCT; {d-2nal}-CY-{d-Trp}-KVCT-NH2 (Disulfide bridge: Cys2-Cys7)
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
1508.2±65.0 °C at 760 mmHg
|
| 闪点 |
865.9±34.3 °C
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
| 折射率 |
1.689
|
| LogP |
2.89
|
| tPSA |
405.68
|
| 氢键供体(HBD)数目 |
13
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
17
|
| 重原子数目 |
77
|
| 分子复杂度/Complexity |
2000
|
| 定义原子立体中心数目 |
9
|
| SMILES |
O=C([C@@H](NC([C@H](C(C)C)NC([C@H](CCCCN)NC([C@@H](CC1=CNC2=C1C=CC=C2)NC([C@H](CC3=CC=C(O)C=C3)N4)=O)=O)=O)=O)CSSC[C@H](NC([C@H](N)CC5=CC=C6C=CC=CC6=C5)=O)C4=O)N[C@@H]([C@H](O)C)C(N)=O
|
| InChi Key |
PUDHBTGHUJUUFI-SCTWWAJVSA-N
|
| InChi Code |
1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74)/t30-,38-,40+,41+,42-,43+,44+,45+,46+/m1/s1
|
| 化学名 |
(4R,7S,10S,13R,16S,19R)-13-((1H-indol-3-yl)methyl)-19-((R)-2-amino-3-(naphthalen-2-yl)propanamido)-N-((2S,3R)-1-amino-3-hydroxy-1-oxobutan-2-yl)-10-(4-aminobutyl)-16-(4-hydroxybenzyl)-7-isopropyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide
|
| 别名 |
Somatulin; Somatuline; Lanreotide; Lanreotide; 108736-35-2; Lanreotida; Ipstyl; Lanreotidum; Autogel; Somatulin-Autogel; UNII-0G3DE8943Y; Ipstyl; BIM23014; Lanreotide Autogel.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9121 mL | 4.5607 mL | 9.1214 mL | |
| 5 mM | 0.1824 mL | 0.9121 mL | 1.8243 mL | |
| 10 mM | 0.0912 mL | 0.4561 mL | 0.9121 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。