| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
| 靶点 |
The target of Muraglitazar (BMS298585) is peroxisome proliferator-activated receptor α (PPARα) and peroxisome proliferator-activated receptor γ (PPARγ), nuclear receptors regulating glucose and lipid metabolism. For human PPARα, the half-maximal effective concentration (EC₅₀) of Muraglitazar is 0.1 μM [2]
; for human PPARγ, the EC₅₀ is 0.03 μM [2] ; it exhibits negligible activation of peroxisome proliferator-activated receptor δ (PPARδ) (EC₅₀ > 10 μM) [2] |
|---|---|
| 体外研究 (In Vitro) |
1. PPAR报告基因激活:在转染PPARα/γ响应性荧光素酶报告质粒的HEK293细胞中,穆格列扎以浓度依赖的方式激活人PPARα和PPARγ,对PPARα的最大激活效率达阳性对照(非诺贝特)的85%,对PPARγ的最大激活效率达罗格列酮的92% [2]
2. 3T3-L1脂肪细胞分化:用0.01–1 μM的穆格列扎处理3T3-L1前脂肪细胞14天,可促进脂肪细胞分化(油红O染色),0.1 μM浓度下脂质积累较溶媒组增加2.5倍;同时通过实时荧光定量PCR检测发现,其可使PPARγ靶基因(aP2、GLUT4)的表达上调3–4倍 [2] 3. 肝细胞脂质代谢:在原代人肝细胞中,0.1 μM的穆格列扎可使细胞内甘油三酯含量降低30%,并使载脂蛋白A-I(HDL合成标志物)的分泌增加40% [2] 4. 脂肪细胞葡萄糖摄取:在分化的3T3-L1脂肪细胞中,0.1 μM的穆格列扎可使胰岛素刺激的2-脱氧葡萄糖摄取效率较溶媒组提高50% [2] |
| 体内研究 (In Vivo) |
1. db/db糖尿病小鼠:每日口服1、3、10 mg/kg的穆格列扎,持续28天,可剂量依赖性降低空腹血糖:分别较溶媒组降低20%、35%和45%;糖化血红蛋白(HbA1c)分别降低1.2%、2.1%和2.8% [2]
2. ZDF大鼠(2型糖尿病模型):每日口服5 mg/kg的穆格列扎,持续12周,可使空腹血糖降低40%、甘油三酯降低50%、高密度脂蛋白胆固醇(HDL-C)升高30%;通过高胰岛素-正糖钳夹实验检测,胰岛素敏感性提高60% [2] 3. 24周临床单药试验:在332例2型糖尿病患者中,每日口服5 mg和10 mg的穆格列扎,HbA1c分别降低0.8%和1.0%(安慰剂组升高0.1%);空腹血糖分别降低2.1 mmol/L和2.5 mmol/L(安慰剂组升高0.3 mmol/L) [1] 4. 临床试验中的调脂效果:5 mg/d的穆格列扎可使甘油三酯降低15%、HDL-C升高10%;10 mg/d可使甘油三酯降低22%、HDL-C升高14%(安慰剂组:甘油三酯升高2%,HDL-C无变化) [1] 5. 临床试验中的体重变化:24周内,5 mg/d组患者平均体重增加2.1 kg,10 mg/d组增加3.2 kg,而安慰剂组仅增加0.3 kg [1] |
| 酶活实验 |
1. PPARα激活实验:将HEK293细胞以5×10⁴个/孔接种于24孔板,转染含PPARα响应元件(PPRE)的荧光素酶报告质粒、人PPARα表达质粒及海肾荧光素酶质粒(内参)。转染24小时后,用系列稀释的穆格列扎(0.001–10 μM)或溶媒处理细胞18小时,制备细胞裂解液,采用双荧光素酶报告基因检测系统测定荧光素酶活性,计算萤火虫荧光素酶/海肾荧光素酶的相对活性,并从剂量-反应曲线中确定PPARα激活的EC₅₀值 [2]
2. PPARγ激活实验:实验流程与PPARα激活实验一致,替换为人PPARγ表达质粒及PPARγ特异性的PPRE-荧光素酶报告质粒,穆格列扎的测试浓度为0.001–10 μM,以罗格列酮为阳性对照,根据相对荧光素酶活性数据计算PPARγ激活的EC₅₀值 [2] |
| 细胞实验 |
1. 3T3-L1脂肪细胞分化实验:将3T3-L1前脂肪细胞以1×10⁵个/孔接种于6孔板,培养至融合。融合后第2天(第0天),用含胰岛素、地塞米松、IBMX的诱导剂联合系列稀释的穆格列扎(0.01–1 μM)或溶媒诱导细胞分化,每2天更换含胰岛素和穆格列扎的新鲜培养基。第14天,用福尔马林固定细胞,油红O染色观察脂滴,并用异丙醇洗脱染料,在510 nm处测定吸光度;同时提取平行孔的总RNA,通过实时荧光定量PCR分析aP2和GLUT4基因的表达 [2]
2. 原代人肝细胞脂质代谢实验:将原代人肝细胞以2×10⁵个/孔接种于胶原包被的培养板,培养48小时后,用0.01–1 μM的穆格列扎或溶媒处理24小时,采用比色法试剂盒检测细胞内甘油三酯含量,通过ELISA定量培养基中载脂蛋白A-I的分泌量 [2] 3. 3T3-L1脂肪细胞葡萄糖摄取实验:将分化的3T3-L1脂肪细胞血清饥饿4小时,再用0.1 μM的穆格列扎或溶媒处理18小时,加入100 nM胰岛素刺激30分钟后,加入2-脱氧-[¹⁴C]-葡萄糖。洗涤并裂解细胞后,通过闪烁计数器检测细胞内放射性,确定葡萄糖摄取效率 [2] |
| 动物实验 |
1. db/db diabetic mouse study: Male db/db mice (8–10 weeks old) are randomized into four groups (n=10 per group): vehicle (0.5% CMC) and Muraglitazar 1, 3, 10 mg/kg/day. The drug is formulated as a 0.5% CMC suspension and administered orally by gavage once daily for 28 days. FBG is measured weekly via tail vein glucometry, and HbA1c is determined by HPLC at the end of the study. Body weight and food intake are monitored daily [2]
2. ZDF rat study: Male ZDF rats (6 weeks old) are assigned to vehicle or Muraglitazar 5 mg/kg/day groups (n=8 per group). The drug is given orally by gavage once daily for 12 weeks. Blood samples are collected monthly to measure FBG, TG, total cholesterol, HDL-C, and LDL-C via clinical chemistry analyzers. Insulin sensitivity is assessed at week 10 using a hyperinsulinemic-euglycemic clamp [2] 3. 24-week clinical trial protocol: Adult patients with type 2 diabetes (HbA1c 7.0–10.0%) are randomized to placebo, Muraglitazar 5 mg/day, or 10 mg/day (n=112, 110, 110 per group) in a double-blind, parallel design. The drug is administered orally once daily with food for 24 weeks. HbA1c, FBG, lipid profiles, body weight, and adverse events are assessed at baseline and every 4 weeks [1] |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Muraglitazar has known human metabolites that include N-((4-Hydroxyphenyl)methyl)-N-((4-methoxyphenoxy)carbonyl)glycine, 9-Hydroxy muraglitazar, 17-Hydroxy muraglitazar, O-Demethyl muraglitazar, and 12-Hydroxy muraglitazar. 1. Oral absorption: Muraglitazar has high oral bioavailability (≈90%) in humans after a single 5 mg oral dose; peak plasma concentrations (Cmax) are reached at 2–4 hours post-dosing [2] 2. Plasma half-life: The terminal plasma half-life (t₁/₂) of Muraglitazar in humans is approximately 24 hours, supporting once-daily dosing [2] 3. Distribution: Muraglitazar has a volume of distribution (Vd) of 120 L in humans and minimally crosses the blood-brain barrier (brain/plasma ratio <0.1) [2] 4. Metabolism: Muraglitazar is primarily metabolized in the liver by CYP3A4 to form inactive hydroxylated metabolites (M1, M2); less than 5% of the parent drug is excreted unchanged [2] 5. Excretion: In humans, ≈70% of the dose is excreted in feces (as metabolites) within 7 days, and ≈20% in urine [2] 6. Plasma protein binding: Muraglitazar is 99.5% bound to human plasma proteins (primarily albumin), with binding independent of concentration in the therapeutic range (0.01–1 μM) [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Clinical adverse events: In the 24-week trial, the most common adverse events with Muraglitazar are peripheral edema (15% at 5 mg, 22% at 10 mg vs. 5% placebo), weight gain (2.1 kg at 5 mg, 3.2 kg at 10 mg vs. 0.3 kg placebo), and headache (10% vs. 8% placebo) [1]
2. Cardiovascular safety: Post-hoc analysis showed an increased risk of major adverse cardiovascular events (MACE) (myocardial infarction, stroke) with Muraglitazar (HR 1.8, 95% CI 1.1–2.9) compared to placebo [2] 3. Animal toxicity: In a 6-month rat study, Muraglitazar 30 mg/kg/day (10× therapeutic dose) causes cardiac hypertrophy (15% increase in heart weight/body weight ratio) and mild hepatic steatosis, which are reversible upon withdrawal [2] 4. Drug-drug interactions: Co-administration with ketoconazole (CYP3A4 inhibitor) increases plasma Muraglitazar concentrations by 1.5-fold; rifampicin (CYP3A4 inducer) decreases concentrations by 40% [2] 5. Hepatic/renal toxicity: No significant increases in serum creatinine or liver enzymes (ALT/AST) are observed with therapeutic doses of Muraglitazar in clinical trials [1] |
| 参考文献 |
|
| 其他信息 |
Muraglitazar is a member of 1,3-oxazoles.
Muraglitazar (Bristol-Myers Squibb/Merck) is a new agent under investigation for the treatment of patients with type 2 diabetes. It belongs to a novel class of drugs that target the peroxisome proliferator-activated receptors, both alpha and gamma subtypes. In addition to improvements in blood glucose and hemoglobin A1c (HbA1c), muraglitazar treatment is associated with a substantial reduction in triglycerides (TGs), an increase in HDL-C, and a modest decrease in LDL-C levels. Muraglitazar is a dual peroxisome proliferator-activated receptor (PPAR) agonist, with hypoglycemic activity. Muraglitazar causes an increase in HDL-C levels, and a decrease in total cholesterol, apolipoprotein B, triglycerides and HbA1c. This agent is associated with an increased risk of adverse cardiovascular events and heart failure. Drug Indication Investigated for use/treatment in diabetes mellitus type 2. Mechanism of Action Muraglitazar is one of the dual peroxisome proliferator-activated receptor (PPAR) agonists. It interacts with both PPAR alpha and gamma receptors. Working through the PPAR gamma receptor, muraglitazar has very potent insulin-sensitizing effects on liver and muscle to lower the blood sugar levels. working through the PPAR alpha receptor, muraglitazar is very potent in terms of lowering the triglycerides and raising the HDL [high-density lipoprotein] cholesterol and converting small dense LDL [low-density lipoprotein] particles to larger, more buoyant particles, so it promotes a very good lipid profile from the standpoint of prevention of atherosclerosis. 1. Muraglitazar (BMS298585) is a dual PPARα/γ agonist developed by Bristol-Myers Squibb, designed to improve insulin sensitivity (PPARγ) and dyslipidemia (PPARα) in type 2 diabetes, addressing metabolic syndrome comorbidities [2] 2. Mechanism of action: Muraglitazar binds to and activates PPARα/γ, regulating gene expression in glucose/lipid metabolism. PPARγ enhances insulin sensitivity in adipose, muscle, and liver; PPARα upregulates fatty acid oxidation and HDL synthesis in the liver [1] 3. Clinical efficacy: In the 24-week trial, 35% (5 mg) and 42% (10 mg) of patients achieved HbA1c <7.0%, vs. 10% with placebo (p<0.001) [1] 4. Regulatory status: Muraglitazar was never approved by the FDA/EMA due to cardiovascular safety concerns, and its development was terminated in 2006 [2] 5. Comparison with other PPAR agonists: Muraglitazar has balanced PPARα/γ activity, showing superior combined glycemic/lipid-lowering effects compared to PPARγ-selective (rosiglitazone) or PPARα-selective (fenofibrate) agents in preclinical models [2] |
| 分子式 |
C29H28N2O7
|
|---|---|
| 分子量 |
516.54182
|
| 精确质量 |
516.19
|
| 元素分析 |
C, 67.43; H, 5.46; N, 5.42; O, 21.68
|
| CAS号 |
331741-94-7
|
| 相关CAS号 |
331741-94-7
|
| PubChem CID |
206044
|
| 外观&性状 |
Solid powder
|
| 密度 |
1.274g/cm3
|
| 沸点 |
736.4ºC at 760 mmHg
|
| 闪点 |
399.2ºC
|
| 折射率 |
1.601
|
| LogP |
4.819
|
| tPSA |
120.12
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
730
|
| 定义原子立体中心数目 |
0
|
| SMILES |
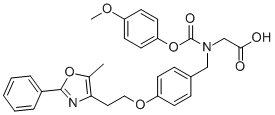
CC1=C(CCOC2=CC=C(C=C2)CN(CC(=O)O)C(=O)OC3=CC=C(C=C3)OC)N=C(C4=CC=CC=C4)O1
|
| InChi Key |
IRLWJILLXJGJTD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H28N2O7/c1-20-26(30-28(37-20)22-6-4-3-5-7-22)16-17-36-24-10-8-21(9-11-24)18-31(19-27(32)33)29(34)38-25-14-12-23(35-2)13-15-25/h3-15H,16-19H2,1-2H3,(H,32,33)
|
| 化学名 |
2-[(4-methoxyphenoxy)carbonyl-[[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]methyl]amino]acetic acid
|
| 别名 |
Muraglitazar; Pargluva; BMS298585; BMS 298585; BMS-298585
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9360 mL | 9.6798 mL | 19.3596 mL | |
| 5 mM | 0.3872 mL | 1.9360 mL | 3.8719 mL | |
| 10 mM | 0.1936 mL | 0.9680 mL | 1.9360 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。