| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
P2Y11 receptor
|
|---|---|
| 体外研究 (In Vitro) |
NF340阻断P2Y11R会抑制IL-1β诱导的基质金属蛋白酶蛋白表达,如MMP-1、MMP-3和MMP-13的水平所示。从机制上讲,阻断P2Y11R可以减轻IL-1β激活的NFκB信号传导,这可以通过减少IκBα磷酸化、核p65积累和NFκB启动子活性来揭示。我们的研究提供了P2Y11R拮抗剂NF340对细胞因子诱导的炎症的保护机制的证据。因此,靶向P2Y11R可能对RA的治疗具有潜在的治疗意义。[1]
阻断P2Y11R抑制IL-1β诱导的TNF-α和IL-6的表达[1] 接下来,我们试图阐明P2Y11R在炎症反应中的作用。我们应用P2Y11R特异性拮抗剂NF340阻断受体的活性,并评估FLS对IL-1β引发的炎症的反应。在mRNA水平上,与未处理的细胞相比,IL-1β诱导了约5.8倍的TNF-α表达。然而,10和20μM NF340阻断P2Y11R后,IL-1β诱导的TNF-α表达分别以剂量依赖的方式改善了3.5倍和2.2倍(图3a)。同样,IL-1β诱导了4.5倍的IL-6 mRNA表达,而在两剂NF340存在的情况下,IL-1β仅诱导了约3倍和1.5倍的IL-6表达(图3a)。我们能够在蛋白质水平上证实这种P2Y11R依赖性抑制作用。同样,与未处理的细胞相比,IL-1β诱导了TNF-α蛋白约五倍的表达。然而,当两种剂量的拮抗剂分别存在于培养基中时,IL-1β仅引起约3倍和1.7倍的TNF-α蛋白诱导(图3b)。同样,IL-1β诱导了约4.2倍的IL-6蛋白表达。然而,当两种剂量的拮抗剂分别存在于培养基中时,IL-1β仅引起IL-6蛋白约2.8倍和1.5倍的表达(图3b)。 阻断P2Y11R抑制IL-1β诱导的氧化应激[1] 然后,我们评估了阻断P2Y11R对细胞因子诱导的细胞应激反应的影响。我们测试了细胞氧化应激的两个方面,包括直接测量ROS和4-HNE的总水平,4-HNE是氧化应激的脂质副产物。与未治疗的FLS相比,IL-1β产生的总细胞ROS水平约为四倍。然而,当培养基中分别存在两剂NF340时,它只导致ROS产生约三倍和两倍(图4a)。与未处理的细胞相比,IL-1β处理诱导的4-HNE产生水平的变化产生了约3.8倍的4-HNE产生。然而,在相同的两剂NF340存在下,它只产生了大约2.5倍和1.8倍的4-HNE产量(图4b)。 阻断P2Y11R抑制IL-1β诱导的MMP表达[1] MMPs活性的增加是RA发展过程中组织重塑的一个重要特征,已经表明MMPs可以被促炎细胞因子诱导。我们测试了P2Y11R阻断对IL-1β诱导的FLS中MMP表达的影响。在mRNA水平上,与未治疗的对照组相比,IL-1β在mRNA转录水平上分别引发MMP-1、MMP-3和MMP-13的平均表达增加了五到六倍。但是,当在培养基中加入10和20μM的NF340来阻断P2Y11R活性时,IL-1β对MMPs的诱导作用仅为两到三倍(图5a)。在蛋白质水平上,与未处理的细胞相比,IL-1β分别引发MMP-1、MMP-3和MMP-13 mRNA蛋白表达平均增加四至五倍。然而,当向培养基中加入相同的两剂NF340时,IL-1β仅诱导平均增加两到三倍(图5b)。MMP表达谱的这些变化表明,细胞因子诱导的FLS中MMPs的表达依赖于P2Y11R的活性。 阻断P2Y11R可减轻IL-1β诱导的NF-κB活化[1] 已知细胞因子诱导的促炎因子在很大程度上依赖于NF-κB的激活。我们测试了P2Y11R被抑制时细胞IκBα激酶磷酸化的状态。与未治疗的FLS相比,IL-1β诱导了IκBα的五倍磷酸化。然而,当使用相同的两剂NF340阻断P2Y11R时,它只导致IκBα磷酸化约3.5倍和2倍(图6)。接下来,我们通过直接检测p65的积累和转染的NF-κB启动子活性来测试核NF-κB的状态。核p65积累是核NF-κB激活的先例事件,因此我们将p65水平的变化与未处理的细胞进行了比较。IL-1β诱导核中p65的积累增加了约3.2倍,但当两种剂量的NF340分别添加到培养基中时,核中的p65仅增加了约2倍和1.5倍(图7a)。当NF-κB荧光素酶启动子转染到FLS中时,其活性在IL-1β处理后增加到约80倍。然而,在两种剂量的NF340存在下,IL-1β诱导的启动子活性的增加分别降低到约50倍和25倍(图7b)。总之,这些实验坚定地证实了P2Y11R活性是细胞因子诱导的NF-κB活化所必需的。 |
| 酶活实验 |
启动子分析[1]
3x NFκB萤光素酶载体购自赛默飞世尔科技公司。使用Lipofectamine 2000试剂将细胞与GL3-Renilla对照质粒和NF-κB-萤火虫报告质粒共转染。转染后24小时,在存在或不存在10和20μMNF340的情况下,用10 ng/mL IL-1β处理细胞24小时。收集总细胞裂解物以测量肾菌和萤火虫荧光素酶的双重荧光素酶活性。通过将萤火虫荧光素酶的活性归一化为肾荧光素酶来计算相对荧光素酶。 |
| 细胞实验 |
细胞处理实验[1]
人FLS在含有10%FBS的完全DMEM培养基中维持。对于细胞因子治疗实验,FLS被铺在6孔板中,并生长到80%至90%的融合。然后用5、10和20 ng/mL的IL-1β处理细胞24小时。为了用NF340处理阻断P2Y11R的活性,将10和20μM浓度的新鲜NF340加入细胞生长培养基中24小时。对照用溶剂DMSO处理。 |
| 参考文献 | |
| 其他信息 |
Rheumatoid arthritis is a common chronic inflammatory joint disease. Fibroblast-like synoviocytes-mediated inflammation is closely associated with the development of rheumatoid arthritis. In this study, we report that P2Y11 receptor activity is required for cytokine-induced inflammation in primary fibroblast-like synoviocytes (FLS). P2Y11R is fairly expressed in primary FLS isolated from healthy subjects and is elevated to around three- to four-fold in rheumatoid arthritis-derived FLS. The expression of P2Y11R is inducible upon IL-1β treatment. Blockage of P2Y11R by its antagonist suppresses IL-1β-induced TNF-α and IL-6 induction and ameliorates oxidative stress as determined by levels of cellular ROS and the oxidative byproduct 4-HNE. Moreover, blockage of P2Y11R by NF340 inhibits IL-1β-induced matrix metalloproteinase protein expression as indicated by the levels of MMP-1, MMP-3, and MMP-13. Mechanistically, blockage of P2Y11R mitigates IL-1β-activated NFκB signaling, which was revealed by reduced IκBα phosphorylation, nuclear p65 accumulation, and NFκB promoter activity. Our study provides evidence of a protective mechanism of P2Y11R antagonist NF340 against cytokine-induced inflammation. Therefore, targeting P2Y11R could have potential therapeutic implication in the treatment of RA. [1]
Researchers conducted a series of inhibition experiments to explore the significance of lowering P2Y11R. First, our experiments show that blockage of P2Y11R by its specific antagonist NF340 indeed weakened IL-1β-induced expression of the pro-inflammatory cytokines TNF-α and IL-6. Second, we show that blockage of this receptor by NF340 reduced IL-1β-induced cellular ROS production and decreased the oxidative stress marker 4-HNE. Third, NF340 appears to potently suppress IL-1β-induced MMP protease activity. NF340 suppresses at least three primary members of the MMP family that indicates that it could hamper the proteolytic erosive activity of FLS, thereby exerting a protective role in joints. It has been reported that FLS are responsible for eroding bone and destroying cartilage through the release of MMPs The suppression of IL-1β induction and MMP activity by NF340 implies that this compound could have therapeutic potential. Indeed, a very recent report showed that 0.3 to 20 μM NF340 reduced a variety of arthritic lesions and improved animal joint conditions after 21 day treatment in an RA rat model, suggesting that NF340 might have additional targets and effects in rats. Several purinergic receptors have been documented to be involved in the pathological development of RA. It has been reported that the P2X7 receptor is expressed by synoviocytes from the joints of RA patients. Activation of the P2X7 receptor stimulates secretion of the pro-inflammatory cytokine IL-6. Deficiency of the P2X7 receptor induced a loss of ATP-dependent leukocyte function, IL-1β production, and L-selectin shedding. Blockage of this receptor has been associated with lower incidence and severity of arthritis in animal models. Interestingly, there exists crosstalk between the P2Y11 receptor and the P2X7 receptor. The P2Y11 receptor can interfere with P2X7 receptor pore formation and thus prevent cell death of human CD8+ T lymphocytes. We found that the expression of P2X7R in RA-FLS was higher than that of the control. Indeed, several preliminary experiments in our laboratory have demonstrated that the P2X7 receptor played a key role in the inflammatory response in FLS (data not shown). Future investigations will provide us with a complete picture of the underlying mechanisms. This study has the following unanswered questions: more detailed understanding of the mechanism of action is required to evaluate the effect of NF340. One of the major problems we face is that the murine genome does not have a human P2Y11R orthologue, and there is no available P2Y11R specific knockout mouse model. Some other preclinical studies were based on rats, but still provided only a limited understanding of this receptor.13 In rats, NF340 has been shown to act on glial cells and participate in the maintenance of neuropathic pain. In human monocyte-derived dendritic cells, NF340 suppresses thrombospondin-1 secretion and reverses LPS stimulated interleukin-12 release. In conclusion, our understanding of the P2Y11R receptor and experience with its antagonist in pharmacological study are still limited. Our study using human FLS provides mechanistic insight into the effect of P2Y11R and its antagonist NF340. Nevertheless, targeting P2Y11R using NF340 could have the potential to be used in clinical trials for RA.[1] |
| 分子式 |
C37H26N4NA4O15S4
|
|---|---|
| 分子量 |
986.83
|
| 精确质量 |
985.986
|
| 元素分析 |
C, 45.03; H, 2.66; N, 5.68; Na, 9.32; O, 24.32; S, 13.00
|
| CAS号 |
202982-98-7
|
| PubChem CID |
90488883
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| tPSA |
362
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
64
|
| 分子复杂度/Complexity |
1850
|
| 定义原子立体中心数目 |
0
|
| SMILES |
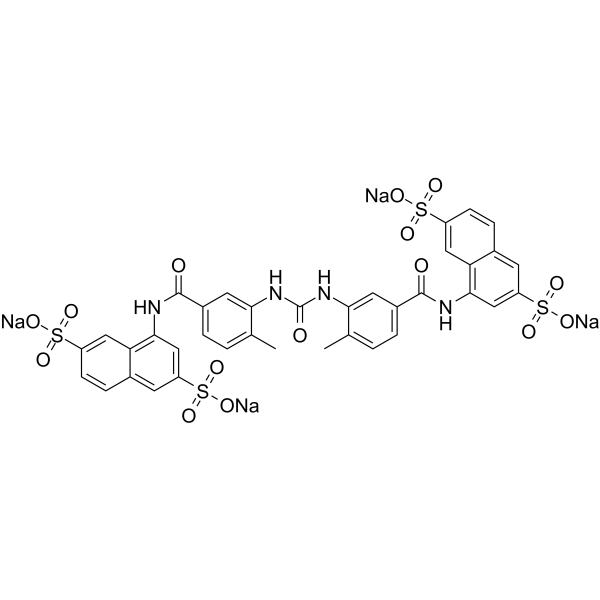
[Na+].[Na+].[Na+].[Na+].O=C(NC1C(C)=CC=C(C(NC2=CC(S([O-])(=O)=O)=CC3C=CC(=CC2=3)S([O-])(=O)=O)=O)C=1)NC1=C(C)C=CC(C(NC2=CC(S([O-])(=O)=O)=CC3C=CC(=CC2=3)S([O-])(=O)=O)=O)=C1
|
| InChi Key |
SJMHXBFWMZYDBY-UHFFFAOYSA-J
|
| InChi Code |
InChI=1S/C37H30N4O15S4.4Na/c1-19-3-5-23(35(42)38-33-17-27(59(51,52)53)11-21-7-9-25(15-29(21)33)57(45,46)47)13-31(19)40-37(44)41-32-14-24(6-4-20(32)2)36(43)39-34-18-28(60(54,55)56)12-22-8-10-26(16-30(22)34)58(48,49)50;;;;/h3-18H,1-2H3,(H,38,42)(H,39,43)(H2,40,41,44)(H,45,46,47)(H,48,49,50)(H,51,52,53)(H,54,55,56);;;;/q;4*+1/p-4
|
| 化学名 |
tetrasodium;4-[[3-[[5-[(3,7-disulfonatonaphthalen-1-yl)carbamoyl]-2-methylphenyl]carbamoylamino]-4-methylbenzoyl]amino]naphthalene-2,6-disulfonate
|
| 别名 |
NF340; NF-340; NF 340; 202982-98-7; tetrasodium;4-[[3-[[5-[(3,7-disulfonatonaphthalen-1-yl)carbamoyl]-2-methylphenyl]carbamoylamino]-4-methylbenzoyl]amino]naphthalene-2,6-disulfonate; 4,4'-(Carbonylbis(imino-3,1-(4-methyl-phenylene)carbonylimino))bis(naphthalene-2,6-disulfonic acid) tetrasodium salt; 4,4'-(Carbonylbis(imino-3,1-(4-methyl-phenylene)carbonylimino))bis(naphthalene-2,6-disulfonicacid)tetrasodiumsalt; NF 340
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0133 mL | 5.0667 mL | 10.1335 mL | |
| 5 mM | 0.2027 mL | 1.0133 mL | 2.0267 mL | |
| 10 mM | 0.1013 mL | 0.5067 mL | 1.0133 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。