| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Natural product
|
|---|---|
| 体外研究 (In Vitro) |
综上所述,(±)-Pantothenic acid是酰基化载体、辅酶a和酰基载体蛋白(ACP)的组成部分。这种维生素很容易从各种饮食来源中获得,这一事实在试图引起(±)-Pantothenic acid缺乏时遇到的困难中得到了强调。虽然(±)-Pantothenic acid缺乏与任何特定的疾病没有联系,但缺乏维生素会导致临床上的全身不适。鉴于(±)-Pantothenic acid是合成辅酶a所必需的,令人惊讶的是,组织中辅酶a水平在(±)-Pantothenic acid缺乏的情况下没有改变。这表明细胞有能力保存其(±)-Pantothenic acid含量,可能是通过利用从含(±)-Pantothenic acid分子降解中获得的(±)-Pantothenic acid的再循环机制。虽然(±)-Pantothenic acid转化为辅酶a的步骤已经被表征,但仍有许多工作要做,以了解辅酶a合成的调节。特别是,考虑到已知的(±)-Pantothenic acid激酶的体外调节,令人惊讶的是,这种酶在体内是活跃的,因为已知的抑制酶的因素超过了已知的抑制酶的浓度。[1]
|
| 体内研究 (In Vivo) |
(±)-泛酸(3 × 200 mg/kg;IP,单次治疗)抑制 VPA 诱导的 c-Myb 和 Pim-1 蛋白表达下降;注射VPA的CD-1小鼠中NTD的发生率下降至6.8%,而未治疗组的发生率为23.6%[1]。
妊娠期间子宫内暴露于丙戊酸(VPA)与神经管缺陷(NTDs)的风险增加有关。尽管VPA介导这些作用的机制尚不清楚,但VPA引发的胚胎蛋白水平变化已被认为是有牵连的。本研究的目的是探讨子宫内VPA暴露对CD-1小鼠胚胎p53、NF-kappaB、Pim-1、c-Myb、Bax和Bcl-2蛋白水平的影响。我们还评估了叶酸和(±)-Pantothenic acid对vpa诱导的NTDs和vpa诱导的胚胎蛋白变化的保护作用。在神经管关闭前给怀孕的CD-1小鼠注射致畸剂量的VPA,并分析胚胎蛋白水平。在我们的研究中,VPA (400 mg/kg)诱导的NTDs(24%)和暴露于VPA的NTD胚胎与表型正常的幼崽相比,p53水平增加了2倍,NF-kappaB、Pim-1和c-Myb蛋白水平降低了4倍(P<0.05)。此外,VPA提高了胚胎Bax/Bcl-2蛋白比值(P<0.05)。在VPA前预处理叶酸或(±)-Pantothenic acid对VPA诱导的NTDs有显著的保护作用(P<0.05)。叶酸还能降低vpa诱导的p53、NF-kappaB、Pim-1、c-Myb和Bax/Bcl-2蛋白水平的改变,而(±)-Pantothenic acid可阻止vpa诱导的NF-kappaB、Pim-1和c-Myb的改变。我们假设叶酸和(±)-Pantothenic acid通过独立但并非相互排斥的机制保护CD-1胚胎免受vpa诱导的NTDs,两者都可能通过预防vpa诱导的参与神经发育的蛋白质改变而介导。[2] |
| 动物实验 |
(±)-Pantothenic acid/VPA treatment[2]
Pregnant mice were grouped as described above with one group treated on GD 9 with a single subcutaneous injection of 400 mg/kg VPA in addition to three intraperitoneal injections of 200 mg/kg of (±)-Pantothenic acid dissolved in PBS (pH 7.4), 1 h prior to the VPA injection, immediately after the VPA injection, and 1 h after the VPA injection. A second treatment group was just given VPA (400 mg/kg) on GD 9, and received the (±)-Pantothenic acid vehicle PBS as described above. The corresponding controls were similarly treated with vehicles alone. For the teratology studies, the numbers of dams analyzed were 6, 5, 5, and 13 for the vehicle control, PTA control, VPA, and VPA + PTA treatment groups, respectively. For analysis of proteins, the number of dams treated varied between 3 and 9 from which embryos were pooled. The FA/PTA dosing regime was based on a study performed by Sato et al., 1995, in which they observed a significant decrease in NTDs using this PTA dosing schedule. |
| 参考文献 | |
| 其他信息 |
Pantothenic acid is a member of the class of pantothenic acids that is an amide formed from pantoic acid and beta-alanine. It has a role as a plant metabolite. It is a conjugate acid of a pantothenate.
DL-Pantothenic acid has been reported in Daphnia pulex, Drosophila melanogaster, and other organisms with data available. See also: Pantothenic Acid (annotation moved to); 4-Deoxypyridoxine (annotation moved to). hus, other physiological regulatory factors (which are largely unknown) must counteract the effects of these inhibitors, since the pantothenate-to-CoA conversion is operative in vivo. Another step in the biosynthetic pathway that may be rate limiting is the conversion of 4'-phosphopantetheine (4'-PP) to dephospho-CoA, a step catalyzed by 4'-phosphopantetheine adenylyl-transferase. In mammalian systems, this step may occur in the mitochondria or in the cytosol. The teleological significance of these two pathways remains to be established, particularly since mitochondria are capable of transporting CoA from the cytosol. Altered homeostasis of CoA has been observed in diverse disease states including starvation, diabetes, alcoholism, Reye syndrome (RS), medium-chain acyl CoA dehydrogenase deficiency, vitamin B12 deficiency, and certain tumors. Hormones, such as glucocorticoids, insulin, and glucagon, as well as drugs, such as clofibrate, also affect tissue CoA levels. It is not known whether the abnormal metabolism observed in these conditions is the result of altered CoA metabolism or whether CoA levels change in response to hormonal or nonhormonal perturbations brought about in these conditions. In other words, a cause-effect relation remains to be elucidated. It is also not known whether the altered CoA metabolism (be it cause or result of abnormal metabolism) can be implicated in the manifestations of a disease. Besides CoA, pantothenic acid is also an integral part of the ACP molecule.[1] |
| 分子式 |
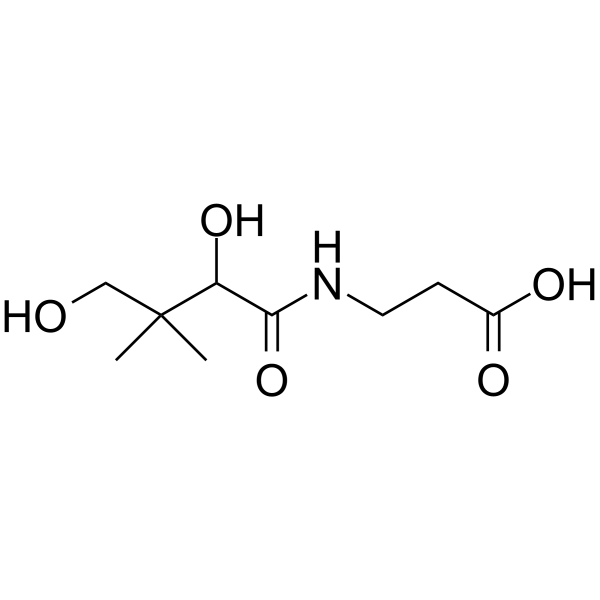
C9H17NO5
|
|---|---|
| 精确质量 |
219.111
|
| CAS号 |
599-54-2
|
| 相关CAS号 |
Pantothenic acid-13C3,15N hemicalcium;356786-94-2
|
| PubChem CID |
988
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.266g/cm3
|
| 沸点 |
551.5ºC at 760mmHg
|
| 闪点 |
287.3ºC
|
| LogP |
-1.1
|
| tPSA |
110.35
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
15
|
| 分子复杂度/Complexity |
239
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(CO)(C(O)C(NCCC(O)=O)=O)C
|
| InChi Key |
GHOKWGTUZJEAQD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C9H17NO5/c1-9(2,5-11)7(14)8(15)10-4-3-6(12)13/h7,11,14H,3-5H2,1-2H3,(H,10,15)(H,12,13)
|
| 化学名 |
3-[(2,4-dihydroxy-3,3-dimethylbutanoyl)amino]propanoic acid
|
| 别名 |
DL-Pantothenic acid; 599-54-2; 3-[(2,4-dihydroxy-3,3-dimethylbutanoyl)amino]propanoic acid; 3-(2,4-dihydroxy-3,3-dimethylbutanamido)propanoic acid; CHEBI:7916; 66Y94D1203; ( inverted exclamation markA)-Pantothenic acid; N-(2,4-dihydroxy-3,3-dimethylbutanoyl)-beta-alanine;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。