| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After oral adminitration, perampanel is absorbed rapidly and completely. Perampanel is eliminated mostely in the feces (48%) and to a lesser exten in the urine (22%). The volume of distribution of perampanel was not quantified. In healthy patients, perampanel has a clearance of about 12 mL/min. Metabolism / Metabolites Perampanel is highly metabolized by CYP3A4 and/or CYP3A5 primary oxidation and by sequential glucuronidation. Biological Half-Life Perampanel has a long elmination half-life of about 105 hours. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Limited data are available on the hepatotoxicity of perampanel. In clinical trials, therapy with perampanel was not associated with an increased frequency of serum aminotransferase elevations as compared to placebo treatment, and there were no reported instances of clinically apparent liver injury. No individual case reports of perampanel hepatotoxicity have been published since its general clinical availability. Perampanel has been implicated in rare instances of severe cutaneous hypersensitivity reactions including DRESS syndrome, which can be associated with variable degrees of liver injury. Thus, clinically apparent liver injury due to perampanel may occur, but must be very rare. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation A minimal amount of information indicates that perampanel milk levels are quite low. If perampanel is required by the mother, it is not a reason to discontinue breastfeeding, but monitor the infant for drowsiness, agitation, adequate weight gain, and developmental milestones, especially in younger, exclusively breastfed infants and when using combinations of drugs. ◉ Effects in Breastfed Infants An infant was exclusively breastfed by a mother taking perampanel, brivaracetam and lacosamide for 6 weeks, then partially breastfed. The infant did not exhibit reduced wakefulness or feeding problems. At one year of age, the mother reported normal development.[1] ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Perampanel is 95-96% plasma protein bound with most binding to the plasma proteins α1-acid glycoprotein and albumin. |
| 参考文献 |
Trinka E, Steinhoff BJ, Nikanorova M, Brodie MJ. Perampanel for focal epilepsy: insights from early clinical experience. Acta Neurol Scand. 2016 Mar;133(3):160-72. doi: 10.1111/ane.12529. Review. PubMed PMID: 26506904; PubMed Central PMCID: PMC4738453.
|
| 其他信息 |
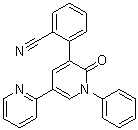
Perampanel is a member of the class of bipyridines that is 2,3'-bipyridin-6'-one substituted at positions 1' and 5' by phenyl and 2-cyanophenyl groups respectively. Used as an adjunctive therapy for the treatment of partial-onset seizures in patients with epilepsy. It has a role as an AMPA receptor antagonist and an anticonvulsant. It is a pyridone, a nitrile and a member of bipyridines. It is functionally related to a benzonitrile.
Perampanel is a noncompetitive AMPA glutamate receptor antagonist. It is marketed under the name Fycompa™ and is indicated as an adjunct in patients over 12 years old for the treatment of partial-onset seizures that may or may not occur with generalized seizures. The FDA label includes an important black-boxed warning of serious or life-threatening behavioral and psychiatric reactions in patients taking Fycompa™. Perampanel is a Noncompetitive AMPA Glutamate Receptor Antagonist. The mechanism of action of perampanel is as an AMPA Receptor Antagonist, and UGT2B7 Inhibitor, and UGT1A9 Inhibitor, and Cytochrome P450 3A4 Inhibitor, and Cytochrome P450 2C8 Inhibitor. Perampanel is glutamate receptor antagonist that is used as an anticonvulsant in the therapy of partial onset seizures. Perampanel has not been associated with serum aminotransferase elevations during therapy, and clinically apparent liver injury from perampanel has yet to be reported and must be rare, if it occurs at all. Perampanel is an orally active, non-competitive, and selective alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor antagonist, with anti-epileptic activity. Although the mechanism of action through which perampanel exerts its antiepileptic effect has not been fully elucidated, this agent antagonizes the AMPA subtype of the excitatory glutamate receptor found on postsynaptic neurons in the central nervous system (CNS). This antagonistic action prevents AMPA receptor activation by glutamate and results in the inhibition of neuronal excitation, repetitive neuronal firing, and the stabilization of hyper-excited neural membranes. Glutamate, the primary excitatory neurotransmitter in the CNS, plays an important role in various neurological disorders caused by neuronal hyperexcitation. See also: Perampanel Hydrate (annotation moved to). Drug Indication Perampanel is indicated for the treatment of partial-onset seizures with or without secondarily generalized seizures in epileptic patients four years of age and older. It is also indicated as an adjunct in the treatment of primary generalized tonic-clonic seizures in epileptic patients aged 12 years and older. FDA Label Fycompa is indicated for the adjunctive treatment of partial-onset seizures with or without secondarily generalised seizures in adult and adolescent patients from 12 years of age with epilepsy. Fycompa is indicated for the adjunctive treatment of primary generalised tonic-clonic seizures in adult and adolescent patients from 12 years of age with idiopathic generalised epilepsy. Treatment of treatment-resistant epilepsies Mechanism of Action The exact mechanism of action of perampanel in seizures is not yet determined, but it is known that perampanel decreases neuronal excitation by non-competitive ihibition of the AMPA receptor. Pharmacodynamics Perampanel is involved in inhibiting neuronal excitation in the central nervous system leading to such effects as decreased pyschomotor performance. |
| 分子式 |
C23H15N3O
|
|---|---|
| 分子量 |
349.38
|
| 精确质量 |
349.121
|
| CAS号 |
380917-97-5
|
| 相关CAS号 |
380917-97-5
|
| PubChem CID |
9924495
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
619.1±55.0 °C at 760 mmHg
|
| 闪点 |
328.2±31.5 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.706
|
| LogP |
3.7
|
| tPSA |
58.68
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
664
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1C(C2=C([H])C([H])=C([H])C([H])=C2C#N)=C([H])C(C2=C([H])C([H])=C([H])C([H])=N2)=C([H])N1C1C([H])=C([H])C([H])=C([H])C=1[H]
|
| InChi Key |
PRMWGUBFXWROHD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H15N3O/c24-15-17-8-4-5-11-20(17)21-14-18(22-12-6-7-13-25-22)16-26(23(21)27)19-9-2-1-3-10-19/h1-14,16H
|
| 化学名 |
5'-(2-cyanophenyl)-1'-phenyl-2,3'-bipyridinyl-6'(1'H)-one
|
| 别名 |
E 2007 E2007 E-2007 ER15505590 ER 15505590 Perampanel Fycompa Related CAS#
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8622 mL | 14.3111 mL | 28.6221 mL | |
| 5 mM | 0.5724 mL | 2.8622 mL | 5.7244 mL | |
| 10 mM | 0.2862 mL | 1.4311 mL | 2.8622 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
PeRampanel fOr Status ePilEpticus pRophylaxis Post-cardiac Arrest
CTID: NCT06401707
Phase: Phase 2 Status: Recruiting
Date: 2024-06-20