| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
|
| 靶点 |
COX-3
|
|---|---|
| 体外研究 (In Vitro) |
非那西丁在抑制COX-3方面比对乙酰氨基酚更有效。在30μM的底物条件下,非那西丁抑制COX-3的IC50值为102μM,而在类似条件下测试的对乙酰氨基酚为460μM。与对乙酰氨基酚一样,非那西丁优先抑制COX-3[1]。
|
| 体内研究 (In Vivo) |
表2显示了长期吸烟对非那西丁在大鼠体内的药代动力学特征的影响。吸烟组和对照组非那西丁的平均血浆浓度-时间曲线如图1A所示。结果表明,经过长期吸烟预处理后,吸烟组非那西丁的AUC(0-∞)、t1/2和Cmax与对照组相比分别显著降低了32%、64%和27%(P<0.05),非那西汀的CL显著增加了35%(P<0.05)。这表明CYP1A2活性是由长期吸烟引起的。[2]
动物实验结果表明,黄芩苷(450mg/kg,静脉注射)显著降低了非那西丁的Cmax和CL,增加了C(60min)、t1/2、Vd和AUC(P<0.05)。在11只大鼠中,非那西丁的C(60分钟)、t1/2、CL、AUC的控制百分比与黄芩苷的Cmax之间存在显著相关性(P<0.05)。体外蛋白结合实验表明,黄芩苷(0-2000mg/L)使未结合的非那西丁从14.5%增加到28.3%。综上所述,黄芩苷可以抑制HLMs中CYP1A2的活性,并表现出与基因多态性无关的较大个体间差异。黄芩苷可以改变非那西丁在大鼠体内的药代动力学。[3] 黄芩苷治疗对非那西丁药代动力学的影响非那西汀的药代动力学:药代动力学研究中获得的非那西坦血药浓度与时间曲线如图3A所示。这清楚地表明,在对照组中,非那西丁的浓度太低,在给药后90分钟无法检测到,而在用黄芩苷治疗的大鼠中,其浓度仍为(0.12±0.02)mg·L-1。如表4所示,黄芩苷(450mg/kg,静脉注射)可显著降低非那西丁的Cmax和CL,并增加C60 min、t1/2、Vd和AUC(P<0.05)。对照组非那西丁的AUC和C60 min分别为(140.2±14.7)mg/L·min和(0.27±0.14)mg/L,而黄芩苷(450 mg/kg)治疗的大鼠分别为(162.8±21.1)mg/L•min和(0.55±0.17)mg/L。联合使用黄芩苷使非那西丁的平均AUC增加了16%,非那西汀的平均C60分钟增加了104%[3]。 |
| 酶活实验 |
药物抑制试验。[1]
Sf9细胞感染高滴度病毒原液(moi=3)并培养48小时。细胞在25°C下与药物预孵育30分钟,然后加入花生四烯酸(100μl,终浓度5或30μM)在37°C下再孵育10分钟。通过放射免疫分析法测定上清液中PGE2的COX活性。检测进行了多次,一式三份。构建抑制曲线,并使用PRISM 3.0测定IC50值。 非那西丁体外大鼠血浆蛋白结合的测定[3] 体外测定黄芩苷对新鲜大鼠血浆(n=5)中非那西丁蛋白结合的影响。 血浆样本中非那西丁的最终浓度为7 mg/L,黄芩苷的浓度在0至2000 mg·L-1之间变化。将样品在37°C下孵育30分钟,然后放入超滤管中。将样品以4500rpm离心15分钟。滤液中非那西丁的浓度通过上述方法测定。 |
| 细胞实验 |
血浆中药物浓度的测量[2]
使用安捷伦1200 HPLC系统进行色谱分析,该系统配备有四元泵、脱气器、自动进样器、恒温柱室和API 4000三重四极仪器(AB/MDSSciex,Ontario,Canada)。 在30°C下,在150 mm×2.1 mm、3.5µm的Agilent Zorbax SB-C18柱上实现了分离。流动相由0.1%甲酸水溶液和乙腈(45:55,v:v)(Merck KGaA,德国)的混合物组成,流速为0.4 mL/min。典型的注射体积为10µL。 通过峰面积法进行定量。目标离子的测定在SIM模式(非那西丁m/z 180,甲磺丁脲m/z 271,氯唑沙宗m/z 167,咪达唑仑m/z 327,IS m/z 237)和正离子电喷雾电离界面下进行。干燥气体流量设置为6 L/min,温度设置为350°C。将系统的雾化器压力和毛细管电压分别调节至20psi和3500V。非那西丁、甲磺丁脲、氯唑沙宗和咪达唑仑的定量限分别为10、20、15和8 ng/mL。[2] 测定了个体HLM中CYP1A2的动力学参数和黄芩苷对CYP1A2作用的IC50。此外,还估计了合并HLM中的Ki值。通过探针底物非那西丁形成对乙酰氨基酚来评估CYP1A2活性。孵育混合物含有不同浓度的HLMs(0.3mg/ml)、100mM磷酸盐缓冲液(pH7.4)、非那西丁和黄芩苷以及NADPH(1mM)。混合物在37°C下预孵育5分钟,最佳孵育时间为30分钟。[3] 对于生物转化,在以下范围内检查了八种底物浓度:非那西丁为6.25至800µM。通过非线性回归分析确定每个HLM的Km和Vmax值。为了估算Ki值,在合并的HLM中使用了不同浓度的非那西丁(12.5、25、50、100、200µM)和黄芩苷(0、10、20、40、80µM)(n=9)。根据CYP1A2基因型和28个HLM的Km值,选择9个HLM。从Lineweaver–Burk图中以图形方式估计了抑制机制。Ki值是通过Lineweaver–Burk图与抑制剂浓度的第二个斜率图计算得出的。此外,底物浓度选择接近Km,并测定黄芩苷对每个HLM中CYP1A2的IC50。[3] 通过加入冰冷的乙腈终止酶反应。非那西丁代谢产物对乙酰氨基酚的测定方法如下。将培养管涡旋并离心,然后将80µl澄清上清液注入HPLC系统。流动相由甲醇和0.05 M乙酸铵(20∶80,v/v)组成,流速为1 ml·min-1。紫外检测波长为257nm。 |
| 动物实验 |
Effects of Baicalin on Phenacetin Pharmacokinetics in Rats in vivo[3]
Sprague–Dawley rats were chosen to conduct this experiment and drug dosing was done via the tail vein. The study was based on a randomized, two-period crossover design at intervals of 4 days. Eleven rats were randomly divided into two groups. Group 1 included 6 rats and group 2 included 5 rats. During the phase I, the rats in group 1 were treated with normal saline (control) and the rats in group 2 were treated with baicalin (450 mg/kg, i.v.). After that an i.v. dose (5 mg/kg) of phenacetin was given immediately. Blood samples were collected before and at 0, 5, 15, 30, 60, 90 and 120 min after administration by orbital bleeding via heparinized capillary tubes. The sample at 0 min was collected immediately after i.v. injection of phenacetin. Plasma was separated from the blood by centrifugation at 4000 rpm for 10 min and was stored at −30°C until analyzed. After a washout period of 4 days, the two groups crossed over to receive the alternative drug. Determination of Plasma Phenacetin and Baicalin Concentration[3] Plasma concentration of phenacetin was determined by HPLC-UV. 1 ml acetic ether was added to 0.1 ml of plasma from each sample and vortexed for 2 min. The samples were centrifuged and the organic phase was evaporated to dryness under a stream of nitrogen. The residue was reconstituted in 100 µl of mobile phase and 50 µl was injected to the HPLC system. The mobile phase consisted of methanol and water (51∶49, v/v) at a flow rate of 1 ml·min−1. The UV detection wavelength was 247 nm. The method of determining plasma baicalin concentration had been reported previously [3]. After complete the modeling, a cocktail solution at a dose of 5 mL/kg, which contained phenacetin (20 mg/kg), tolbutamide (5 mg/kg), chlorzoxazone (20 mg/kg) and midazolam (10 mg/kg) in CMC-Na solution, was administered orally to all rats in each group. Blood samples of each rat were collected as the following times: pre-dose (0 h) and then at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48 h after probe drugs administration through the tail vein and immediately separated by centrifugation at 13,000 rpm for 10 min to obtain plasma. The total volume of blood taken from each animal did not exceed 2.2 mL. A total of 100 µL plasma samples were transferred to a new tube and stored frozen at −80 °C until analyzed. Rats of smoking group and control group (n=4) were killed. Each liver sample was quickly removed and store at −80 °C[2]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
... Oral absorption of phenacetin is markedly influenced by particle size in the preparation, & plasma concentration of phenacetin & acetaminophen are correspondingly variable. Peak concentration of phenacetin in plasma usually occurs in about 1 hr, & that of acetaminophen derived there from in 1-2 hr. Absorption following oral administration is rapid ... duration of effect is about 4 hr. Up to 45% of (14)C was recovered in 16 hr urine & 1% in feces of rats given [acetyl-(14)C]phenacetin per oral. For more Absorption, Distribution and Excretion (Complete) data for PHENACETIN (9 total), please visit the HSDB record page. Metabolism / Metabolites Metabolised in the body to paracetamol. Acetaminophen & phenacetin are metabolized primarily by hepatic microsomal enzymes. ... In normal individual, 75 to 80% of administered phenacetin is rapidly metabolized to acetaminophen. ... Phenacetin is converted to at least a dozen other metabolites, by n-deacetylation to para-phenetidin & by hydroxylation & further metabolism of phenacetin & para-phenetidin. An unknown metabolite, but an oxidizing agent, is responsible for methemoglobin formation & hemolysis of red blood cells ... . Phenacetin is metabolized ... to p-acetamidophenol, which is excreted as glucuronide and sulfate conjugate ... . ... N-hydroxyphenacetin has been identified as metabolite in ... man. For more Metabolism/Metabolites (Complete) data for PHENACETIN (15 total), please visit the HSDB record page. Phenacetin has known human metabolites that include N-Hydroxyphenacetin and acetaminophen. Metabolised in the body to paracetamol. Biological Half-Life The elimination half-life (t1/2)beta varied from 37 to 74 minutes. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Phenacetin's analgesic effects are due to its actions on the sensory tracts of the spinal cord. In addition, phenacetin has a depressant action on the heart, where it acts as a negative inotrope. It is an antipyretic, acting on the brain to decrease the temperature set point. It is also used to treat rheumatoid arthritis (subacute type) and intercostal neuralgia.
Toxicity Data
Acute oral toxicity (LD50): 866 mg/kg [Mouse].
Toxin and Toxin Target Database (T3DB)
12.1.6 Interactions
Sodium 3-hydroxy-4-iodo-2-naphthoate and sodium 1-hydroxy-4-bromo-naphthoate inhibited phenacetin absorption in the rat intestine. The blood, brain and kidney levels of phenacetin decreased and the liver level increased in rats following simultaneous oral administration of phenacetin and either one. The blood levels of phenacetin in rabbits were decreased by both but were increased by sodium 1-hydroxy-2-naphthoate, sodium tetrahydro-1-hydroxy-2-naphthoate, and sodium tetrahydro-3-hydroxy-2-naphthoate. All derivatives tested decreased the in vitro metabolism of phenacetin by rat or rabbit liver slices.
Niwa H et al; Tohoku Yakka Daigaku Kenkyu Nempo 18: 1 (1971)
Hazardous Substances Data Bank (HSDB)
The transformation of acetophenetidin to n-acetyl-p-aminophenol was increased in the liver from rats treated with caffeine or antipyrine. The lung and intestine were also capable of metabolizing acetophenetidin to form this metabolite, and this pathway was increased by exposure to cigarette smoke. Following a test dose of acetophenetidin to human subjects, the plasma levels of acetophenetidin were lower in people who smoke cigarettes than in nonsmokers. Thus, in addition to the liver, the lung and intestine may have important roles in the metabolism of acetophenetidin.
|
| 参考文献 |
[1]. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002 Oct 15;99(21):13926-31.
[2]. Effects of long-term smoking on the activity and mRNA expression of CYP isozymes in rats. J Thorac Dis. 2015 Oct; 7(10): 1725–1731. [3]. Inhibition of Baicalin on Metabolism of Phenacetin, a Probe of CYP1A2, in Human Liver Microsomes and in Rats. PLoS One. 2014; 9(2): e89752. [4]. https://en.wikipedia.org/wiki/Phenacetin |
| 其他信息 |
Phenacetin can cause cancer according to California Labor Code.
Phenacetin is an odorless fine white crystalline solid with a lightly bitter taste. Used as an analgesic medicine. Phenacetin is a member of the class of acetamides that is acetamide in which one of the hydrogens attached to the nitrogen is substituted by a 4-ethoxyphenyl group. It has a role as a non-narcotic analgesic, a peripheral nervous system drug and a cyclooxygenase 3 inhibitor. It is a member of acetamides and an aromatic ether. It is functionally related to a N-phenylacetamide, a 4-ethoxyaniline and a paracetamol. Phenacetin was withdrawn from the Canadian market in June 1973 due to concerns regarding nephropathy (damage to or disease of the kidney). Phenacetin is a synthetic, white crystalline solid that is slightly soluble in water and benzene, soluble in acetone and very soluble in pyrimidine. It is used in research as the preferred marker for detecting CYP1A2-based inhibition potential in vitro. Human ingestion of phenacetin can result in a bluish discoloration of the skin due to a lack of oxygen in the blood (cyanosis), dizziness and respiratory depression. It is reasonably anticipated to be a human carcinogen. (NCI05) Phenacetin was withdrawn from the Canadian market in June 1973 due to concerns regarding nephropathy (damage to or disease of the kidney). A phenylacetamide that was formerly used in ANALGESICS but nephropathy and METHEMOGLOBINEMIA led to its withdrawal from the market. (From Smith and Reynard, Textbook of Pharmacology,1991, p431) See also: Aspirin; Butalbital; Caffeine (annotation moved to). Drug Indication Used principally as an analgesic. Mechanism of Action The present study was aimed to test the possible cyclooxygenase (COX)-1/COX-2 selectivity of the old analgesic drug phenacetin and its metabolite p-phenetidine, which exhibits high renal toxicity. Paracetamol (acetaminophen), the main metabolite of phenacetin with low renal toxicity, and indomethacin were selected as reference compounds. Collagen-stimulated platelet thromboxane B2 (TxB2) production and phorbol 12-myristate-13-acetate (PMA)-induced neutrophil prostaglandin E2 (PGE2) synthesis were used as indicators for COX-1 and COX-2 activity, respectively. Phenacetin was even less potent than paracetamol to reduce the production of both TxB2 and PGE2, and no clear preference for either of the COX-enzymes was seen. P-phenetidine was a more potent inhibitor, already at nanomolar level, of the synthesis of these prostanoids than indomethacin and showed some preference to COX-2 inhibition. Somewhat higher, micromolar, concentrations of p-phenetidine also reduced COX-2 expression in neutrophils. We suggest that the very potent inhibitory activity of p-phenetidine on PGE2 synthesis combined with the reduction of COX-2 expression could explain the renal papillary necrosis in phenacetin kidney. Analgesic nephropathy is a unique drug-induced kidney disease characterized pathologically by renal papillary necrosis and chronic interstitial nephritis, and is the result of excessive consumption of combination antipyretic analgesics. The clinical features of the disorder relate mainly to the papillary necrosis, renal colic, and obstructive uropathy and the development of chronic renal failure in a small percentage of patients. There are significant geographic variations in the clinical features that may be related to the differing combinations of analgesics. The pathogenesis of the disease is in part related to the kidneys' ability to concentrate drugs in the papillae. The following sequence of events presents a plausible explanation for the evolution of the disease. If a combination of phenacetin and aspirin is ingested, the following steps occur. Phenacetin is converted in the gut and liver to acetaminophen by first-pass metabolism. Acetaminophen is then taken up by the kidney and excreted. During its excretion, acetaminophen becomes concentrated in the papillae of the kidney during physiologic degrees of antidiuresis, the concentration being up to five times the intracellular concentration of other tissues. Acetaminophen undergoes oxidative metabolism by prostaglandin H synthase to a reactive quinoneimine that is conjugated to glutathione. If acetaminophen is present alone, there is sufficient glutathione generated in the papillae to detoxify the reactive intermediate. If the acetaminophen is ingested with aspirin, the aspirin is converted to salicylate and salicylate becomes highly concentrated in both the cortex and papillae of the kidney. Salicylate is a potent depletor of glutathione. The mechanism is not completely understood; however, the inhibition of the production of NADPH via the pentose shunt is a possible explanation. With the cellular glutathione depleted, the reactive metabolite of acetaminophen then produces lipid peroxides and arylation of tissue proteins, ultimately resulting in necrosis of the papillae. The mechanism of analgesic action has not been fully determined. Acetaminophen may act predominantly by inhibiting prostaglandin synthesis in the central nervous system (CNS) and, to a lesser extent, through peripheral action by blocking pain impulse generation. The peripheral action may also be due to inhibition of of the synthesis or actions of other substances that sensitive pain receptors to mechanical or chemical stimulation. /Acetaminophen/ Acetaminophen probably produces antipyresis by acting centrally on the hypothalamic heat-regulating center to produce peripheral vasodilation resulting in increased blood flow through the skin, sweating, and heat loss. The central action probably involves inhibition of prostaglandin synthesis in the hypothalamus. /Acetaminophen/ |
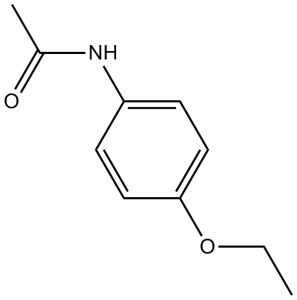
| 分子式 |
C10H13NO2
|
|
|---|---|---|
| 分子量 |
179.22
|
|
| 精确质量 |
179.094
|
|
| 元素分析 |
C, 67.02; H, 7.31; N, 7.82; O, 17.85
|
|
| CAS号 |
62-44-2
|
|
| 相关CAS号 |
Phenacetin;62-44-2
|
|
| PubChem CID |
4754
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.0±0.1 g/cm3
|
|
| 沸点 |
323.6±44.0 °C at 760 mmHg
|
|
| 熔点 |
133-136 °C(lit.)
|
|
| 闪点 |
149.5±28.4 °C
|
|
| 蒸汽压 |
0.0±0.7 mmHg at 25°C
|
|
| 折射率 |
1.506
|
|
| LogP |
2.01
|
|
| tPSA |
38.33
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
13
|
|
| 分子复杂度/Complexity |
162
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
CPJSUEIXXCENMM-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C10H13NO2/c1-3-13-10-6-4-9(5-7-10)11-8(2)12/h4-7H,3H2,1-2H3,(H,11,12)
|
|
| 化学名 |
N-(4-Ethoxyphenyl)acetamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.5797 mL | 27.8987 mL | 55.7973 mL | |
| 5 mM | 1.1159 mL | 5.5797 mL | 11.1595 mL | |
| 10 mM | 0.5580 mL | 2.7899 mL | 5.5797 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。