| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
GT5a (EC50 = 1.4 pM); GT1a H77 (EC50 = 1.8 pM); GT2b (EC50 = 1.9 pM); GT4a (EC50 = 1.9 pM); GT3a (EC50 = 2.1 pM)
Pibrentasvir is a potent pan-genotypic next-generation HCV NS5A inhibitor that retains activity against common amino acid substitutions of HCV genotypes 1–6, which are known to confer resistance to NS5A inhibitors that are currently approved.[1] |
|---|---|
| 体外研究 (In Vitro) |
Pibrentasvir 是一种有效的泛基因型下一代 HCV NS5A 抑制剂,保留了针对 HCV 基因型 1-6 常见氨基酸取代的活性,已知这些氨基酸取代会对目前批准的 NS5A 抑制剂产生耐药性。 [1]
匹布瑞韦对包含基因型1至6 NS5A的稳定HCV亚基因组复制子表现出强效、全基因型的抗病毒活性。在0%人血浆中,50%有效浓度值在1.4至5.0 pM之间:针对基因型1a-H77为1.8 pM,1b-Con1为4.3 pM,2a-JFH-1为5.0 pM,针对包含基因型2a、2b、3a、4a、5a和6a NS5A的嵌合复制子,EC50在1.4至2.8 pM之间。 由于血浆蛋白结合,在40%人血浆存在下,匹布瑞韦的抗病毒活性减弱了35至47倍,EC50分别增加到64 pM和200 pM。 匹布瑞韦对一组包含来自HCV感染患者样本的NS5A基因的复制子显示出相似的活性,这些样本涵盖基因型1a、1b、2a、2b、3a、4a、5a和6a/e/p,中位EC50在0.50至2.7 pM之间。这些样本包括在与其他NS5A抑制剂耐药相关位点存在基线多态性的样本。 匹布瑞韦对一系列已知可导致对其他NS5A抑制剂耐药的单一位点NS5A氨基酸替换保持了完全活性,这些替换存在于基因型1至6中,EC50倍数变化通常≤7。例如,针对基因型1a的单替换:M28T, Y93H, Y93N。针对基因型3a的Y93H替换,其EC50仅增加了2.3倍。 它还保留了对包含NS3/4A蛋白酶抑制剂和NS5B聚合酶抑制剂关键耐药相关替换的复制子的活性,EC50倍数变化≤1.7,表明无交叉耐药。 匹布瑞韦对人类免疫缺陷病毒1型或乙型肝炎病毒没有可测量的抗病毒活性。 在基因型1b复制子细胞中进行的棋盘法联合研究及MacSynergy II分析表明,当匹布瑞韦与干扰素-α、利巴韦林或HCV NS3/4A蛋白酶抑制剂格卡瑞韦合用时,产生轻微至中度的协同抗病毒活性。未观察到拮抗作用。 [1] |
| 体内研究 (In Vivo) |
研究提到,匹布瑞韦强效的体外活性转化为在为期3天的单药治疗临床试验中强大的抗病毒活性。在感染基因型1、未经治疗的成人中,每日40至400 mg的剂量在3天结束时使HCV血浆RNA从基线平均最大下降≥4.1 log₁₀ IU/ml。选择用于3期研究的120 mg剂量实现了4.5 log₁₀ IU/ml的下降。在此单药治疗研究中,40名患者中仅有3名出现了NS5A耐药相关替换。 [1]
|
| 细胞实验 |
南方研究所开展针对 HBV 和 HIV-1 的抗病毒活性测定。 Pibrentasvir 在 HIV-1 抗病毒细胞保护试验中使用 IIIB 病毒株和 CEM-SS 细胞进行评估。简而言之,将病毒和细胞混合,并在 pibrentasvir 或齐多夫定(AZT;阳性对照)存在下孵育六天。病毒滴度是预先确定的,因此感染病毒的对照孔显示出 85% 至 95% 的细胞活力因病毒复制而丧失。因此,当化合物阻碍病毒复制时,就会观察到抗病毒作用或细胞保护作用。感染六天后,向每个孔中添加二十至二十五微升的MTS试剂,然后将微量滴定板孵育四至六小时,以测量细胞的活力。使用 Molecular Devices Vmax 或 SpectraMax Plus 读板器在 490/650 nm 处以分光光度法读取板。
稳定HCV复制子细胞中的抗病毒活性:培养稳定的HCV亚基因组复制子细胞系。细胞在含有5%胎牛血清、有或无40%人血浆的培养基中与匹布瑞韦的系列稀释液孵育3天。通过使用发光计测量细胞裂解液中的荧光素酶报告基因活性来确定HCV复制抑制。使用非线性回归曲线拟合计算EC50。 细胞毒性实验:在Huh-7细胞、HepG2细胞和MT4细胞中评估细胞毒性。将细胞接种于96孔板中并与匹布瑞韦孵育。孵育一段时间后,使用MTT比色法测量细胞活力。计算50%细胞毒性浓度。匹布瑞韦在Huh-7细胞中的CC50 >32,000,000 pM,在HepG2和MT4细胞中>10,000,000 pM,表明治疗指数很高。 抗HIV-1和HBV活性实验:对于HIV-1,使用抗病毒细胞保护实验。CEM-SS细胞在匹布瑞韦存在下感染HIV-1,孵育6天。使用MTS试剂评估细胞活力,保护作用表明抗病毒效应。对于HBV,用匹布瑞韦处理HepG2 2.2.15细胞6天。蛋白酶处理后,通过实时定量PCR对培养上清液中的细胞外HBV DNA进行定量。根据HBV DNA水平的降低计算EC50。 耐药选择实验:将含有特定基因型NS5A的HCV稳定复制子细胞接种,并在G418和浓度为其EC50 10倍或100倍的匹布瑞韦存在下培养。每3-4天更换一次培养基,持续约3周。挑取存活克隆并扩大培养,通过RT-PCR扩增其NS5A编码区并进行测序,以鉴定耐药相关的氨基酸替换。计算克隆存活率。 突变体敏感性瞬时复制子实验:构建在NS5A、NS3或NS5B中带有特定氨基酸替换的工程化HCV复制子。将质粒线性化,转录成RNA,并转染到Huh-7细胞中。通过荧光素酶实验测量匹布瑞韦对这些HCV复制子复制的抑制。计算复制效率,表示为野生型复制的百分比。 联合研究:在基因型1b-Con1复制子细胞中,使用棋盘法将匹布瑞韦与另一种HCV抑制剂以系列2倍稀释的方式进行组合。通过荧光素酶报告基因实验确定HCV复制。使用MacSynergy II程序分析数据,计算协同/拮抗体积并确定相互作用类型。 [1] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In healthy subjects, the time it takes to reach the peak plasma concentration (Tmax) is approximately 5 hours. The mean peak plasma concentration (Cmax) is 110ng/mL in non-cirrhotic HCV-infected subjects. Relative to fasting conditions, the consumption of meals increases the absorption of pibrentasvir by 40-53%. The predominant route of elimination of the drug is biliary-fecal, where 96.6% of administered drug is excreted in feces and 0% of the drug is excreted in the urine. Metabolism / Metabolites Pibrentasvir is not metabolized. Biological Half-Life The elimination half life (t1/2) is approximately 13 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Pibrentasvir has not been studied in nursing mothers being treated for hepatitis C infection. Because it is >99.9% bound to maternal plasma proteins, amounts in breastmilk are likely to be very low. Hepatitis C is not transmitted through breastmilk and breastmilk has been shown to inactivate hepatitis C virus (HCV). However, the Centers for Disease Control recommends that mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. It is not clear if this warning would apply to mothers who are being treated for hepatitis C. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Pibrentasvir is >99.9% bound to human plasma proteins. The Blood-to-plasma ratio is approximately 0.62. The study provides in vitro cytotoxicity data (CC50), as described in the Cell Assay section, indicating low cellular toxicity. [1] |
| 参考文献 | |
| 其他信息 |
Pibrentasvir is a direct acting antiviral agent and Hepatitis C virus (HCV) NS5A inhibitor that targets the the viral RNA replication and viron assembly. In combination with [DB13879], pibrentastiv is a useful therapy for patients who experienced therapeutic failure from other NS5A inhibitors. In cell cultures, the emergence of amino acid substitutions at known NS5A inhibitor resistance-associated positions in HCV genotype 1a, 2a or 3a replicons led to reduced susceptibility and resistance to pibrentasvir. These resistance-associated amino acid substitutions included Q30D/deletion, Y93D/H/N or H58D +Y93H in genotype 1a replicons, F28S + M31I or P29S + K30G in genotype 2a replicons, and Y93H in genotype 3a replicons. Individual NS5A amino acid substitutions that reduced susceptibility to pibrentasvir include M28G or Q30D in a genotype 1a replicon and P32-deletion in a genotype 1b replicon. Pibrentasvir is available as an oral combination therapy with [DB13879] under the brand name Mavyret. This fixed-dose combination therapy was FDA-approved in August 2017 to treat adults with chronic hepatitis C virus (HCV) genotypes 1-6 without cirrhosis (liver disease) or with mild cirrhosis, including patients with moderate to severe kidney disease and those who are on dialysis. Mavyret is also indicated for HCV genotype 1-infected patients who have been previously treated with regimens either containing an NS5A inhibitor or an NS3/4A protease inhibitor, but not both. Hepatitis C viral infection often leads to decreased liver function and subsequent liver failure, causing a significantly negative impact on the patients' quality of life. The ultimate goal of the combination treatment is to achieve sustained virologic response (SVR) and cure the patients from the infection. In clinical trials, this combination therapy achieved SVR12 rate, or undetectable Hepatitis C for twelve or more weeks after the end of treatment, of ≥93% across genotypes 1a, 2a, 3a, 4, 5 and 6.
Pibrentasvir is a Hepatitis C Virus NS5A Inhibitor. The mechanism of action of pibrentasvir is as a P-Glycoprotein Inhibitor, and Breast Cancer Resistance Protein Inhibitor, and Organic Anion Transporting Polypeptide 1B1 Inhibitor, and Organic Anion Transporting Polypeptide 1B3 Inhibitor, and Cytochrome P450 3A Inhibitor, and Cytochrome P450 1A2 Inhibitor, and UGT1A1 Inhibitor. Drug Indication Indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A). MAVYRET is also indicated for the treatment of adult patients with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both. FDA Label Mechanism of Action NS5A is a phosphoprotein that plays an essential role in replication, assembly and maturation of infectious viral proteins. The basal phosphorylated form of NS5A, which is maintained by C-terminal serine cluster, is key in ensuring its interaction with the viral capsid protein, or the core protein. By blocking this interaction, pibrentasvir inhibits the assembly of proteins and production of mature HCV particles. NS5A also interacts with viral and cellular proteins to form the HCV replicase complex, and supports the RNA replication of HCV. Pharmacodynamics Pibrentasvir is a pan-genotypic . According to HCV replicon assays, pibrentasvir has EC50 values ranging from 0.08-4.6 nM agaisnt laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4d, 5a, and 6a, or EC50 values of 0.5-4.3 pM against laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4b, 4d, 5a, 6a, 6e and 6p. It is active against common resistance-conferring substitutions in HCV genotypes 1 to 6 that confers resistance and decreased therapeutic response from other NS5A inhibitors, inluding positions 24, 28, 30, 31, 58, 92, or 93 in NS5A. In a QT study, pibrentasvir is not shown to prolong the QTc interval. Pibrentasvir is a novel, next-generation HCV NS5A inhibitor with a chemical name provided in the Materials and Methods. Its mechanism of action is inhibition of the HCV NS5A protein, which plays multiple critical roles in viral RNA replication and virion assembly. The study highlights pibrentasvir's high genetic barrier to resistance, as evidenced by the low frequency of colony selection in vitro and the low rate of emergent resistance in a short-term monotherapy clinical trial. Pibrentasvir has been co-administered with glecaprevir (an HCV NS3/4A protease inhibitor) in clinical studies. This combination achieved high sustained virologic response rates in treatment-naive non-cirrhotic patients infected with HCV genotypes 1-6, and in genotype 1-infected patients who had failed a prior direct-acting antiviral regimen. [1] |
| 分子式 |
C57H65F5N10O8
|
|---|---|
| 分子量 |
1113.2
|
| 精确质量 |
1112.49
|
| 元素分析 |
C, 61.50; H, 5.89; F, 8.53; N, 12.58; O, 11.50
|
| CAS号 |
1353900-92-1
|
| 相关CAS号 |
1821461-48-6 (butanamine hydrate);1353900-92-1 (free);
|
| PubChem CID |
58031952
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.614
|
| LogP |
8.71
|
| tPSA |
199.58
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
17
|
| 可旋转键数目(RBC) |
17
|
| 重原子数目 |
80
|
| 分子复杂度/Complexity |
2000
|
| 定义原子立体中心数目 |
8
|
| SMILES |
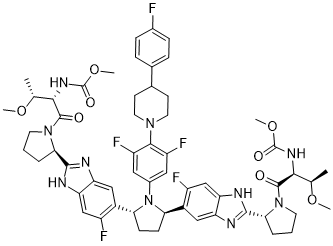
FC1C=C2C(=CC=1[C@H]1CCC(C3=C(C=C4C(=C3)NC([C@@H]3CCCN3C([C@H]([C@@H](C)OC)NC(=O)OC)=O)=N4)F)N1C1C=C(C(=C(C=1)F)N1CCC(C3C=CC(=CC=3)F)CC1)F)NC([C@@H]1CCCN1C([C@H]([C@@H](C)OC)NC(=O)OC)=O)=N2
|
| InChi Key |
VJYSBPDEJWLKKJ-NLIMODCCSA-N
|
| InChi Code |
InChI=1S/C57H65F5N10O8/c1-29(77-3)49(67-56(75)79-5)54(73)70-19-7-9-47(70)52-63-41-25-35(37(59)27-43(41)65-52)45-15-16-46(72(45)34-23-39(61)51(40(62)24-34)69-21-17-32(18-22-69)31-11-13-33(58)14-12-31)36-26-42-44(28-38(36)60)66-53(64-42)48-10-8-20-71(48)55(74)50(30(2)78-4)68-57(76)80-6/h11-14,23-30,32,45-50H,7-10,15-22H2,1-6H3,(H,63,65)(H,64,66)(H,67,75)(H,68,76)/t29-,30-,45-,46-,47+,48+,49+,50+/m1/s1
|
| 化学名 |
methyl N-[(2S,3R)-1-[(2S)-2-[6-[(2R,5R)-1-[3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl]-5-[6-fluoro-2-[(2S)-1-[(2S,3R)-3-methoxy-2-(methoxycarbonylamino)butanoyl]pyrrolidin-2-yl]-3H-benzimidazol-5-yl]pyrrolidin-2-yl]-5-fluoro-1H-benzimidazol-2-yl]pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl]carbamate
|
| 别名 |
ABT-530; ABT 530; ABT530; A-1325912; A1325912; A 1325912; A 1325912.0; A1325912.0; A-1325912.0; Pibrentasvir
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.8983 mL | 4.4916 mL | 8.9831 mL | |
| 5 mM | 0.1797 mL | 0.8983 mL | 1.7966 mL | |
| 10 mM | 0.0898 mL | 0.4492 mL | 0.8983 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05637879 | Not yet recruiting | Drug: Glecaprevir/pibrentasvir Other: Placebo |
PTSD | White River Junction Veterans Affairs Medical Center |
July 1, 2023 | Phase 2 Phase 3 |
| NCT04575896 | Active Recruiting |
Drug: Glecaprevir/pibrentasvir | End Stage Renal Disease Hepatitis C |
Johns Hopkins University | November 20, 2020 | Phase 4 |
| NCT04903626 | Recruiting | Drug: Glecaprevir/Pibrentasvir (GLE/PIB) |
Hepatitis C Virus (HCV) |
AbbVie | August 24, 2021 | Phase 3 |
| NCT05446857 | Recruiting | Drug: Glecaprevir/Pibrentasvir Pill | PTSD | White River Junction Veterans Affairs Medical Center |
April 1, 2023 | Phase 2 Phase 3 |
| NCT03855917 | Recruiting | Drug: Sofosbuvir 400mg [Sovaldi] Drug: Glecaprevir/pibrentasvir (300mg/120mg) |
Hepatitis C | Kirby Institute | February 11, 2020 | Phase 4 |