| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
5-HT4 receptors
|
|---|---|
| 体外研究 (In Vitro) |
1.在体外和体内评估了新型拮抗剂SDZ 205-557(2-甲氧基-4-氨基-5-氯苯甲酸2-(二乙氨基)乙酯)与5-HT3和5-HT4受体的相互作用。2.在豚鼠海马中,在0.4微M 5-甲酰胺色胺的存在下,5-羟色胺介导的腺苷酸环化酶刺激被SDZ 205557竞争性拮抗,pA2值为7.5,Schild斜率为0.81。在卡巴胆碱收缩的大鼠食管中,5-HT4受体介导的舒张作用被具有相似pA2值的SDZ 205557可克服地拮抗(7.3)。该值与激动剂无关,但(R)-扎可必利除外,其值明显较低(6.4)。3.在5-HT3受体的功能研究中,SDZ 205557在豚鼠回肠中的亲和力为6.2,而在NG108-15细胞中[3H]-喹帕津标记的结合位点上的亲和力为6.9[2]。
豚鼠海马腺苷酸环化酶[2] 5-羟色胺(2倍)刺激豚鼠海马分离膜中的腺苷酸环化酶活性(基础活性=1.8 nmol 30分钟-“mg-”蛋白),其-log EC50值为6.4(图1)。5-羟色胺的S型浓度反应曲线似乎是单相的。相比之下,5-甲酰胺色胺(5-CT)产生了双相反应曲线。低浓度(0.4pM)5-CT足以最大限度地刺激初始阶段(图1)。先前的研究表明,在0.4 IM 5-CT存在下,5-HT引起的腺苷酸环化酶活性的增加是由于5-HT4受体的刺激(Shenker等人,1987)。在没有5-CT的情况下,最大有效浓度(100 gM)的SDZ 205-557和spiperone分别将10 LM 5-HT的刺激减少了44%和64%。当膜制剂在两种拮抗剂存在下孵育时,5-HT对腺苷酸环化酶的刺激被消除(图2)。在5-CT存在的情况下,托烷司琼和SDZ 205557以浓度依赖的方式拮抗了对5-HT的反应,其-log ICm值分别为6.4和5.1(平均值,s.e.mean<10%)。相比之下,普萘洛尔(0.1 mM)、酮色林(1 LM)或甲塞菌肽(1 gLM;数据未显示)对5-HT的反应不受影响。通过Schild回归分析得出SDZ 205557在介导腺苷酸环化酶刺激的5-HT4受体上的表观亲和力。pA2值为7.5,Schild斜率为0.81(图3)。 |
| 体内研究 (In Vivo) |
在麻醉的迷走神经切断微量ig中,SDZ 205-557仅对5-HT4介导的心动过速产生短暂阻断。这与托烷司琼形成鲜明对比,托烷司酮在给药后活性超过60分钟。SDZ 205557和托烷司琼的抑制反应半衰期分别为23和116分钟。4.总之,SDZ 205557对5-HT3和5-HT4受体具有相似的亲和力。在豚鼠中观察到的明显选择性是由于该物种中5-HT3受体的非典型性。这种新型拮抗剂的作用时间短可能会使其在体内的使用复杂化。因此,在定义5-HT4受体的研究中,应适当谨慎地使用SDZ 205557[2]。
大鼠食管粘膜肌层[2] 在用10jM卡巴胆碱预收缩的制剂中,所有激动剂都诱导了浓度依赖性舒张。表1显示了所研究的激动剂的效力和内在活性。排序为5-羟色胺=5-MeOT>BIMU-1)SC-53116>BIMU-8>(S)-扎可必利>(R)-扎可必利。SDZ 205-557(1 nM-10pM)在食管5-HT4受体上缺乏内在活性,因为没有观察到舒张作用。在SDZ 205557存在的情况下,所有激动剂的浓度反应曲线以竞争的方式向右移动。使用SDZ 205557(1-10tM)并使用5-HT作为激动剂进行的Schild回归分析表明,Schild斜率与单位没有显著差异(1.24,95%置信区间0.98-1.50)(图4)。当施加单位约束时,确定pA2值为7.3(95%置信区间:7.2-7.4)。单浓度SDZ 205557与其他激动剂的表观亲和力(-log KB)与该值相似(表1)。例外的是当(R)-扎可必利用作激动剂时的亲和力。在这种情况下,-log KB值明显较低。托烷司琼(3 1M)拮抗5-羟色胺诱导的舒张作用,浓度比为35(95%置信区间,27-45),表观亲和力(-log KB)为7.0±0.04。SDZ 205557(3 gM)以98(41-237)的浓度比拮抗对5-HT的反应。在SDZ 205557(3 1M)和托烷司琼(3 1M”)存在的情况下,对5-HT的反应向右移动,合并浓度比为153(99.8-234;平均值,95%置信区间)。这与两种竞争性拮抗剂在同一位点相互作用的组合所预测的结果没有显著差异(即133倍)。 S-HT3受体的相互作用[2] 在豚鼠离体回肠中,在甲塞菌肽(1M)和5-甲氧基色胺(10M)的存在下进行了选择性脱敏5-HTI、5-HT2和5-HT4受体的实验。在回肠5-HT3受体上,SSDZ 205-557(10 ILM)拮抗了对5-HT的反应,表观亲和力(-log KB)为6.2±0.08。Eglen等人(1990)报告的其他5-HT3拮抗剂在类似条件下的亲和力被纳入比较(表2)。因此,回肠5-HT3受体拮抗剂亲和力的排名顺序为:(S)-扎可必利>托烷司琼>昂丹司琼>SDZ 205557。在结合研究中,在NG108-15细胞膜中由[H]-喹嗪鉴定的5-HT3受体上(表3),SDZ 205557置换等温线产生的表观亲和力(-log Kj)为6.9±0.2。Hill系数(1.3±0.1)与unity没有显著差异,表明SDZ 205557在同质群体中相互作用。表观亲和力的排名顺序为(S)-扎可必利>昂丹司琼>托烷司琼>MDL 72222>SDZ 205557>胃复安。结合位点的估计值大于功能研究中的估计值(表2),这与Butler等人(1990)的先前报告一致。 麻醉猪心动过速[2] 5-羟色胺、氯氮平和(R,S)-扎可必利在麻醉猪中引发了剂量依赖性心动过速,其中5-羟色胺的效力明显强于苯甲酰胺(图5)。初步研究表明,猪对5-羟色胺的连续、可重复的剂量反应曲线可以以90-120分钟的间隔构建。每次服用5-羟色胺后10-15分钟内,心率反应恢复到基线,连续服用苯甲酰胺后,反应逐渐累积。5-羟色胺和(R,S)-扎可必利作为完全激动剂,扎可普利德作为完全激动药,氯氮平作为部分激动剂(表3)。对所有这些激动剂的反应都被托烷司琼拮抗(数据未显示)。为了评估托烷司琼和SDZ 205-557的抑制活性,单剂量拮抗剂与ED54剂量的5-羟色胺(3-10tgg kg-',静脉注射)相比,之前是通过单独滴定获得的。对照组和治疗组的差异不足以利用与基线相比的排名变化;因此,使用参数重复测量方差分析来分析这些数据。在这些总体方差分析中,治疗、时间和治疗x时间相互作用的影响都非常显著(P<0.0001)。随后的成对对比(单因素方差分析)表明,静脉注射SDZ 205557 6.0 mg kg-'后,仅在给药后3分钟内显著抑制了对5-HT的心动过速反应(P<0.05),而在实验的其余时间里没有观察到显著效果。相比之下,与类似剂量的5-羟色胺相比,静脉注射托烷司琼(5 mg kg-')在给药后120分钟内显著抑制了反应(P<0.05)(图6)。SDZ 205557和托烷司琼的抑制反应半衰期分别为23(17-35)和116(85-175)分钟(值为95%置信区间的平均值)。 |
| 酶活实验 |
竞争性放射性配体结合研究[2]
膜由在先前描述的条件下培养的NG108-15细胞制备(Sharif等人,1991)。在竞争性结合研究中,NG108-15细胞膜中的5-HT3受体用0.5 nM[3H]-喹嗪标记,非特异性结合由1 JAM(S)-扎可必利定义。将膜匀浆在25 mM Tris-Krebs(250℃下pH 7.4)中与放射性配体和不同浓度的SDZ 205-557或其他标准5-HT3受体拮抗剂一起孵育,最终测定体积为0.5 ml。在250℃下孵育45分钟,并使用Brandell 48细胞采集器在Whatman GF/B过滤器上快速真空过滤终止孵育。随后立即用冰冷的0.1M NaCl洗涤8秒。过滤器用0.3%聚乙烯亚胺预处理,以减少放射性配体的过滤器结合,并通过液体闪烁光谱法测定过滤器上保留的放射性。如前所述,所有比赛数据均通过迭代曲线拟合程序进行分析(Michel&Whiting,1984)。竞争配体的表观离解常数(K)通过Cheng-Prusoff方程从ICso值计算得出(Cheng&Prusoff,1973)。 |
| 动物实验 |
Guinea-pig hippocampal adenylyl cyclase [2]
Male guinea-pigs (Hartley, 250-500 g) were killed by CO2 asphyxiation. The hippocampi were then rapidly dissected, and homogenized (20 strokes) in a glass homogenizer in 9 ml of ice-cold buffer, of the following composition (mM): sucrose 300, Tris-HCl 20, EGTA 1, Na2EDTA 2.5 and dithiothreitol 1 (pH 7.4, 23°C). This homogenate was then diluted, 1:8 (v/v) with buffer and centrifuged (10 min, 39,000 g, 4C). The pellet was resuspended in 9 ml of buffer and the membrane suspension used directly in the adenylyl cyclase assay. Antagonists were added 30 min prior to addition of agonist. Measurements of adenylyl cyclase activity were performed, three times, according to the method of Alvarez & Daniels (1990) with each sample run in triplicate. The final composition of the incubation medium was (mM): [at32P]-adenosine 5'-triphosphate ([&32P]-ATP, 0.25 pCi) 0.5, MgSO4 5, TrisHCl 44 (pH 7.4), 1-methyl-3-isobutylxanthine 1.0, sucrose 50, EDTA 1.0, EGTA 0.2, dithiothreitol 0.2, adenosine 3':5'- cyclic monophosphate (cyclic AMP) 2, guanosine 5'-triphosphate (GTP) 0.1, phosphoenol pyruvate 20 and pyruvate kinase 6 units ml-'. The reaction was initiated by the sequential addition of the membrane suspension (40 #Al) to the incubation medium in a total reaction volume of 200 Jl. Incubations were performed for 30 min at 370C and terminated by the addition of 20 ftl of a stock solution of [3H]-cyclic AMP (0.005 JCi) in 2.2 N HCl. Labelled cyclic AMP was added to estimate and correct for recovery of the nucleotide following column chromatography. The tube contents were heated at 95C for 4 min and then cooled in an ice-water bath. Neutral alumina (1.3 g) was dispensed into disposable polypropylene columns with a Uniflow adjustable powder measure. The columns were placed on a plexiglas rack designed to hold the columns and to fit over a box of 100 scintillation vials. An aliquot (200 pl) of the solution contained in each tube was pipetted onto the columns and allowed to flow into the dry alumina. Cyclic AMP was then eluted with 4 ml of 0.1 M ammonium acetate, pH 7. The effluent (3.2 ml) was collected into scintillation vials, mixed with 15 ml scintillation fluid and counted for 3H and 32p in a liquid scintillation spectrometer. [2] Rat isolated oesophageal muscularis mucosae [2] The method used was that previously described by Baxter et al. (1991). Male rats (Sprague-Dawley, 200-250 g) from Charles River were killed by CO2 asphyxiation, the thoracic oesophagus removed and placed in Tyrode solution (composition, mM: NaCl 139.0, KCl 2.7, MgCl2 6H2O 1.1, NaH2PO4 0.4, glucose 5.6, NaHCO3 11.8 and CaCl2-6H2O 1.8). The outer proprial muscle coat was cut longitudinally and gently peeled away, leaving the inner muscularis mucosae. Silk threads were then tied through the lumen on both ends of the tissues, which were then mounted vertically in 10 ml tissue baths containing Tyrode solution with 1 JAM methysergide, 30 gM cocaine and 30 JM corticosterone. The baths were maintained at 37C and constantly aerated with a mixture of 95% 02 and 5% CO2. An initial resting tension of 1 g was applied to the tissues, then adjusted to 0.5 g tension at 15 min intervals thereafter. After 1 h of equilibration, 100 JM pargyline was added to the baths for 30 min, followed by a 15 min washout. At this point, the tissues were exposed to 50 mM KCl for 5 min, washed four times, followed by an additional 30 min equilibration period with a 15 min wash cycle. Carbachol (3 ,.M) was added to the baths to contract the preparations. Once a stable contraction had been established (usually 30 min) the agonist was then added cumulatively to the bath to induce relaxation. After establishing the control concentration-response curve for the agonist, the preparations were then washed. SDZ 205-557 (1-10IOM) was applied to the baths and allowed to equilibrate with the tissues for 60 min before the second agonist concentration-response curve. Since all 5-HT4 agonists at high concentrations have the propensity to antagonize muscarinic receptors (Baxter et al., 1991), a final concentration-response curve was constructed in the presence of both 10 JM 5-methoxytryptamine and SDZ 205,557 after a further 60 min. This procedure established the agonist concentration-range attributable to 5-HT4 agonism alone as distinct from additional relaxation due to muscarinic receptor antagomsm. Agonist potencies were determined by nonlinear iterative curve fitting procedures (Michelson et al., 1992), using the relationship described by Parker & Waud (1971). Apparent antagonist affinities (- log KB) were estimated by the relationship - log KB = - log [Antagonist]/(concentration ratio - 1) (Furchgott, 1972). Concentration ratios were measured at the agonist concentration which elicited 30% of the maximal relaxation, since under some conditions the effects observed at higher concentrations may have reflected muscarinic receptor antagonism. Against 5-HT itself, the apparent affinity was determined by the method of Arunlakshana & Schild (1959), wherein three concentrations of SDZ 205-557 were used, and the slope of the Schild plot determined by regression analysis. [2] Anaesthetized micropig studies [2] The method used was modified from that described by Villalon et al. (1990). Yucatan micropigs (male and female; 14.9-20.8 kg) were pretreated with ketamine HCl (approx. 30 mg kg-', i.m.), anaesthetized with pentobarbitone sodium (20 mg kg-') via a marginal ear vein, intubated, and mechanically ventilated with room air by an animal respirator. A femoral artery was cannulated for the measurement of arterial blood pressure via a Gould/Statham pressure transducer (P231D). Dual cannulae were inserted into the ipsilateral femoral vein, one cannula for continuous infusion of supplemental anaesthetic (pentobarbitone sodium 8- 15 mg kg-' h-') and the second cannula for compound administration. A limblead II ECG was monitored via subcutaneously placed electrodes and heart rate (HR) was determined by a cardiotachometer triggered by the aortic pressure signal form. Following a midline cervical incision, the vagus nerves were bilaterally transected. Blood gas parameters were periodically monitored via a blood gas analyzer and blood gas values were stabilized within a normal (pH 7.2; Pco2, 33 mmHg; Po2, 96 mmHg) physiological range by adjustments of ventilatory rate, tidal volume, and positive endexpiratory pressure prior to continuing an experiment. 5-HT, tropisetron and SDZ 205-557 were administered in base equivalent doses. 5-HT, dissolved in 0.154 M NaCI, was administered at bolus i.v. doses of 1, 3, 10, 30 and 1I00 g kg-' (0.05 ml kg-' doses) in each animal and a doseresponse curve constructed. An ED50 dose for 5-HT was determined visually from each dose-response curve. SDZ 205,557 and tropisetron were dissolved in a 3:7 propylene glycol:normal saline (v/v) mixture. Animals were assigned randomly to 3 treatment groups: vehicle (3:7 propylene glycol: normal saline), SDZ 205,557 (6 mg kg- , i.v.), or tropisetron (5 mg kg-', i.v.). Doses for SDZ 205-557 and tropisetron were determined in preliminary dose-finding experiments (data not shown). These doses represented those that maximally antagonized 5-HT-induced tachycardia and/ or were the maximum feasible dose based on compound supply and micropig weight. The ED50 dose of 5-HT (determined from a previous experiment) was administered 3 times at 10-15 min intervals to determine a control response (mean of 3 responses). Following administration of vehicle, SDZ 205,557, or tropisetron in a volume of 0.1 ml kg-l, the ED50 dose of 5-HT was administered in a volume of 0.03 ml kg-' at 3, 10, 20, 30, 45, 60, 75, 90, 105, and 120 min thereafter. [2] |
| 参考文献 | |
| 其他信息 |
In the anaesthetized, vagotomized micropig, 5-HT elicited a tachycardic response in the presence of 5-HT1, 5-HT2, 5-HT3, M2-muscarinic and P-adrenoceptor blockade. These responses were antagonized by high doses of tropisetron and mimicked by (R,S)-zacopride and renzapride. Similar observations have been reported by Villalon et al. (1990) in the Yorkshire pig and concur with biochemical data showing that porcine myocardial 5-HT4 receptors stimulate adenylyl cyclase (Kaumann, 1990). The tachycardia may be due to subsequent activation of a kinase (Kaumann et al., 1991) and closure of potassium channels (Bockaert et al., 1992). 5-HTinduced tachycardia in the micropig, thus, provides a suitable in vivo assay to study 5-HT4 compounds. The administration of SDZ 205-557 failed to antagonize, except briefly (3 min) after injection, the tachycardic responses to 5-HT. The doses of 5-HT were submaximal and adequately blocked by tropisetron. The short-lived antagonism by SDZ 205,557 in vivo contrasts with the sustained antagonism seen in vitro (at least 60 min; see Methods). The compound, at least at the dose tested, appears to be subject to rapid metabolism, probably due to hydrolysis at the ester moiety in the SDZ 205-557 molecule. It should be noted, however, that such rapid degradation may not be so marked at higher doses and additional experiments are required to study this. In conclusion, SDZ 205,557 acted as a surmountable 5- HT4 receptor antagonist in guinea-pig hippocampus and rat oesophagus, although it had no selectivity between mouse neuroblastoma 5-HT3 and 5-HT4 receptors. In guinea-pig, a 5-HT4/5-HT3 selectivity was evident due to the atypical nature of the guinea-pig 5-HT3 receptor. The short duration of action in vivo together with this limited selectivity, suggests that caution should be exercised in its use to define the 5-HT4 receptor. [2]
|
| 分子式 |
C14H21CLN2O3
|
|---|---|
| 分子量 |
300.78
|
| CAS号 |
137196-67-9
|
| PubChem CID |
5191
|
| 外观&性状 |
Typically exists as solids at room temperature
|
| 密度 |
1.177g/cm3
|
| 沸点 |
436.8ºC at 760mmHg
|
| 闪点 |
217.9ºC
|
| 蒸汽压 |
7.88E-08mmHg at 25°C
|
| LogP |
3.813
|
| tPSA |
64.79
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
20
|
| 分子复杂度/Complexity |
300
|
| 定义原子立体中心数目 |
0
|
| SMILES |
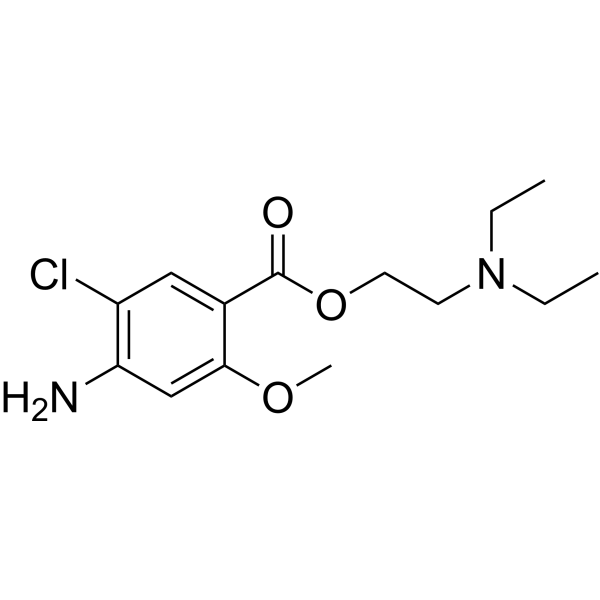
CCN(CCOC(C1=CC(Cl)=C(N)C=C1OC)=O)CC
|
| 别名 |
2-(Diethylamino)ethyl 4-amino-5-chloro-2-methoxybenzoate; SDZ 205-557; SDZ 205557; SDZ-205-557; 2-Methoxy-4-amino-5-chlorobenzoic acid 2-(diethylamino)ethyl ester; Benzoic acid, 4-amino-5-chloro-2-methoxy-, 2-(diethylamino)ethyl ester; DTXSID90160102; ...; 137196-67-9;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.3247 mL | 16.6234 mL | 33.2469 mL | |
| 5 mM | 0.6649 mL | 3.3247 mL | 6.6494 mL | |
| 10 mM | 0.3325 mL | 1.6623 mL | 3.3247 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。