| 规格 | 价格 | |
|---|---|---|
| 500 U | ||
| Other Sizes |
| 靶点 |
Choline ester
|
|---|---|
| 体外研究 (In Vitro) |
在体外和计算机模拟中研究了双酚A(BPA)和双酚S(BPS)这两种常见污染物对电鳗Acetylcholinesterase/乙酰胆碱酯酶(AChE)活性的抑制作用,尤其是在海洋和淡水中。两者都产生了完全的非竞争性抑制作用,但BPA的Ki值是BPS的一半。分子对接分析表明,两者都与活性位点峡谷中部和入口处的PAS和ABS区域中的残基W286、F297、Y337、F338相互作用,并且BPS也与催化三元结构的S203具有氢键。这两种化合物的IC50值在pH:8.2时出现拐点,这表明窄谷中的Y124和/或Y337是结合的主要结构因素。对BPS的抑制效果较差,特别是在25-30°C(酶活性达到峰值的温度)时,这归因于窄谷的构象。AChE的同源性分析最初揭示了显著的同一性,特别是α/β水解酶结构域,它也包括活性位点,与来自不同环境的七种不同硬骨鱼物种的序列。最后,首次发现BPS和BPA一样,是AChE的重要抑制剂,这一点通过在不同pH和温度水平下进行的体外和计算机分析得到了证实。结论是,这种效应也可能适用于大多数其他硬骨鱼的AChE[1]。
|
| 酶活实验 |
乙酰胆碱酯酶活性测定:评估抑制模式[1]
乙酰胆碱酯酶(AChE)活性采用Ellman方法进行测量,并进行了一些修改(Ellman等人,1961)。使用纯电鳗乙酰胆碱酯酶(AChE),同时使用碘化乙酰硫代胆碱作为反应底物。5,5′-二硫代双(2-硝基苯甲酸)酸(DTNB)与巯基发生反应,被称为Elman试剂。硫酸根阴离子与Ellman试剂相互作用,该反应产生一种混合的二硫化物(R-s-TNB−)和2-硝基-5-硫代苯甲酸根阴离子(TNB2−),其吸收412 nm的光。100 mM,pH:8.0,磷酸缓冲液用于反应组、空白组和对照组。在平底96孔微孔板中测试了0.17 mM DTNB和三种不同的乙酰硫代胆碱碘化物底物浓度(0.15、0.25和0.35 mM),以评估BPA和BPS在0.3至3.3 mM范围内的七种不同浓度下的抑制作用。每种浓度的每次试验都制备为三份(3孔),并设计了一个特定的空白作为第四孔。所有这些测量都在不同的日期独立重复了四次。在添加酶时开始活性测量,并在25°C、412 nm下以30秒的间隔测量ABS,检测酶活性10分钟。通过使用以下方程式(方程式(1))计算比活性值 [1] 其中dA/dt是在412nm处测量的吸光度值的变化率,ε是乙酰胆碱酯酶的消光系数,其值为13.6 mM−1 cm−1,DF是稀释因子。 将比活性值提交给许可软件SigmaPlot 13.0酶动力学模块,以检测抑制类型。接受具有最高R2值的软件建议的抑制模式。此外,酶动力学图,如Michaelis-Menten、Lineveawer-Burk、Dixon等,用于分析、演示和证明。 乙酰胆碱酯酶AChE酶的同源性调查[1] 乙酰胆碱酯酶是生物体代谢中必不可少的酶,具有共同的结构,特别是在相同的分类单位中。该蛋白质的硬骨鱼物种的所有定义和RefSeq合格的氨基酸序列都来自NCBI蛋白质数据库,并且都经过MEGA7比对,以评估它们之间的相似性并进行距离分析。MEGA7分配产生了一个系统发育树,并指出了来自其他硬骨鱼物种的相同酶的相同/相似的蛋白质结构/氨基酸残基。这些系统发育树和比对报告由Jalview 2.11.1.4软件观察。根据包含最相同/最相似序列的集群,还讨论了集群中的鱼类及其自然栖息地,以推断它们对Acetylcholinesterase/乙酰胆碱酯酶/乙酰胆碱酯酶具有相似抑制作用的可能性。 |
| 参考文献 | |
| 其他信息 |
The inhibitory effects of BPA and BPS on AChE were determined for seven different concentrations of these two xenobiotics applied in a wide range, and both the inhibition model and kinetic parameters were calculated with statistical accuracy. BPS caused smaller decrease in specific activity values, at all equal concentrations of xenobiotics, with respect to BPA. Moreover, the Ki value of BPA is about half of that of BPS (p < 0.05); which means the inhibitory effect of BPA is considerably higher concerning BPS. On the other hand, the proposed binding energies of both are very close to each other for the best docking position (Supplementary material Tables 1 and 2), which might explain the similarity of binding sites. [1]
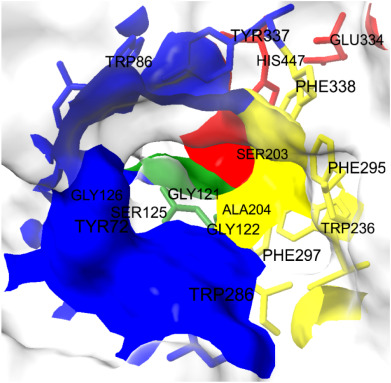
Molecular docking analyses support in vitro experiments and the predicted inhibition model of the full non-competitive type. BPA and BPS interact with amino acids midway through the narrow gorge, especially in the PAS and ABS regions. It has been proposed that PAS facilitates the transport of ACh toward the catalytic triad, hence improving the catalytic performance of Acetylcholinesterase/AChE; that is, ACh binds to PAS and then diffuses quickly down to the catalytic site. This notion is bolstered by the fact that AChE is one of the most efficient biocatalysts in terms of kinetics, and its action kinetics are mostly restricted by diffusion. A critical residue that should be emphasized in the PAS region is W286 (Pourshojaei et al., 2019). Hydrophobic interactions between bisphenol derivatives and the indole group of this tryptophan play a key role in the binding of these two xenobiotics to PAS. Besides, ABS has some vital functions in positioning ACh via some hydrophobic interactions of aromatic side chains of certain amino acids. Phenylalanines at the positions of 295/297/338 perform such a function in natural metabolism. The binding of relatively hydrophobic BPA and BPS to those hydrophobic residues was expected and clearly screened by molecular docking issues. Thus, inhibiting both PAS and ABS of acetylcholinesterase not only slows the rate-limiting phase (diffusion of ACh to the active site) but may also diminish the enzyme's turnover number. Consequently, it is possible that the investigated chemicals could function as blockers of the typical ionic substrate (ACh) entry into the active site gorge. The influence of pH on the inhibitory potencies of BPA and BPS was investigated to reveal the enzyme's ionizable group(s) that contribute to their binding. Both showed similar curves, but the amount of the shift was most significant for BPS, indicating that the binding strongly depends on the acid/base characteristics of a specific amino acid, whose side chain may interact directly with bisphenol derivatives. The measured transition for BPS corresponds to a pKa of 8.099 ± 0.002. Although identifying the ionizable groups responsible for such a change is difficult, the inflection point around pH: 8.1 reveals that a Tyr residue appears to aid in BPS binding. This residue, likely Y124 and/or Y337, might be the key structural component resulting in relatively tight binding. In addition to all these, the structural and functional integrity of the enzyme at different pH values, especially the narrow gorge, should also be taken into account. Ser (125/203), Glu (202/334), Asp (74), Arg (296), His (447) and Tyr (72/124/133/337) residues, which are located both in this channel and around the active site and are involved in directing the substrate to the catalytic triad, undergo changes that will change the binding of both the substrate and other substances in acidic and alkaline environments outside the physiological pH range. As expected, the use of modeling procedures and specialized algorithms that can account for structural changes that occur during inhibitor interaction, particularly in channel-shaped proteins and in enzymes with channel-shaped active sites, like Acetylcholinesterase/AChE, is critical for achieving more accurate findings. The current study determined that the optimal temperature for the Acetylcholinesterase/AChE enzyme is between 25 and 30 °C. In addition, for both BPA and BPS, after this interval, a statistically significant decrease is observed in the IC50 values calculated at the applied temperature of 35 °C. It can be argued that this breaking point is due to a change in the three-dimensional structure of the enzyme. Because of the collective dynamics of conserved amino acids at the gorge, AChE transits between “open” and “closed” conformations. At room temperature, AChE's substantial molecular flexibility governs access to the active site and binding to ligands (Silva et al., 2020). BPS was detected to be affected by this situation more apparently because its IC50 values shifted more than BPA; and it can be suggested that the hydrophobic and pi-pi interactions of BPS with the residues in the catalytic triad and with the amino acids at the bottom of the 20 angstrom-long gorge, which are also occupied by the natural substrate ACh - such as S203, might be responsible. Acetylcholinesterases are characterized by the presence of the α/β hydrolase domain, in addition to some other sites. α/β hydrolase family is a functionally diverse superfamily containing esterases, peroxidases, dehalogenases, epoxide hydrolases, lipases and proteases (Bauer et al., 2020). The catalytic apparatus usually consists of three residues: a serine, a glutamate or aspartate, and a histidine. The mechanism frequently includes a nucleophilic attack on a carbonyl carbon atom. COBALT submission of the sequences stated that there is about 100 % identity at α/β hydrolase domain for the enzymes of the species in the closest cluster (Supplementary material Tables 3 and 4, Supplementary material Figs. 1–17), which means the mode and the magnitude of the inhibition by BPA and BPS might be similar for those other Acetylcholinesterase/AChEs of fishes and electric eel. This shows that the monitoring of BPA and BPS concentrations and kinetic parameters expressing their inhibition on AChE could also be used for different fish species from different ecosystems so that the list contains fish species from tropical to subtropical regions, from North and South America to Asia, and, finally from freshwater to brackish and salt water. It also reveals that the argument that BPS is harmless enough to replace BPA must be reconsidered within the framework of different species, geography, climatic conditions and habitat. BPA is a dangerous and common pollutant, which is found primarily in plastic materials, in almost all ecosystems of the world. BPS was presented as an environmentally-friendly alternative to BPA, and it has a wide usage area. This study was aimed to reveal the inhibitory action of those two chemicals on acetylcholinesterase of a teleost fish species: electric eel. in vitro and in silico analysis and calculations completed in the current project put forward the inhibitory effects of those xenobiotics on this particular enzyme. BPS couldn't be valued as a non-toxic alternative because of its comparatively close Ki and IC50 values with BPA. Moreover, they apply their inhibitory effect on the AChE/Acetylcholinesterase enzyme in the same mode (non-competitive reversible) and in similar ways; so that the interacting residues at the narrow gorge were stated as similar or the same, which was also supported by the almost equal binding energies for the best docking position of each. Further, when the AChE protein sequences of teleost fishes were aligned, very high percentages of identity at critical functional parts such as the narrow gorge in α/β hydrolase domain suggested observing possibly similar inhibitory effects of BPA and BPS on AChEs of most other teleost fish species from diverse ecosystems. Taking account of the fact that there were α/β hydrolase domains in some other enzymes and proteins in various living organisms, BPA and BPS inhibition becomes much more important in the evaluation of their harmful effects on biota. [1] |
| CAS号 |
9000-81-1
|
|---|---|
| 外观&性状 |
White to off-white solid powder
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: 100 mg/mL
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。