| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
RDC Linker for Radionuclide-Drug Conjugates
|
|---|---|
| 体内研究 (In Vivo) |
在原位环境中用氯屈膦酸盐脂质体预处理的小鼠在早期时间点表现出肝脏摄取减少(24小时时为12.2±2.3%ID/g对22.8±3.8%ID/g),在120小时时肿瘤摄取增加(13.8±8.0%ID/g对6.0±1.2%ID/g)。这使得用氯屈膦酸钠治疗的小鼠(6/6)的原位胰腺异种移植物的描绘明显多于未用氯屈磷酸钠治疗的鼠(2/6)或注射用非特异性抗体标记的金纳米粒子的小鼠(0/5)。
结论:氯屈膦酸盐脂质体和金纳米颗粒表面活性靶向抗体的组合允许对小鼠皮下和原位胰腺异种移植物进行PET/CT成像[1]。
|
| 细胞实验 |
细胞培养:[1]
胰腺癌症细胞系BxPC-3和MiaPaCa-2维持在37°C和5%CO2气氛下。BxPC-3细胞在罗斯威尔公园纪念研究所(RPMI)-1640中培养,含有2 mM L-谷氨酰胺、10 mM HEPES、1 mM丙酮酸钠、4.5 g/L葡萄糖、1.5 g/L碳酸钠和10%FBS。MiaPaCa-2细胞在补充了10%FBS和2.5%马血清的Dulbecco改良鹰培养基(DMEM)中培养。 [89Zr]Zr-Ab-AuNP的体外评估:[1] 将1x106细胞接种在1mL培养基中的六孔板上,并在37°C和5%CO2的培养箱中粘附过夜。第二天,向每个孔中加入1 uCi的[89Zr]Zr-5B1-AuNP,并将其放回培养箱中1、4、12和24小时。在每个时间点收集培养基,用磷酸缓冲盐水洗涤孔两次。通过用冷的05 M甘氨酸缓冲液(pH=2.8)洗涤孔两次,然后在4°C下用PBS冲洗,收集表面结合部分。通过用1M NaOH裂解细胞,然后用PBS洗涤两次来收集内化部分。然后在Wizard2自动伽马计数器上对所有分数进行计数. |
| 动物实验 |
Purpose: Targeted delivery in vivo remains an immense roadblock for the translation of nanomaterials into the clinic. The greatest obstacle is the mononuclear phagocyte system (MPS), which sequesters foreign substances from general circulation and causes accumulation in organs such as the liver and spleen. The purpose of this study was to determine whether attaching an active targeting antibody, 5B1, to the surface of gold nanoparticles and using clodronate liposomes to deplete liver and splenic macrophages could help to minimize uptake by MPS organs, increase targeted delivery to CA19.9-positive pancreatic tumors, and enhance pancreatic tumor delineation.
Procedures: To produce the antibody-gold nanoparticle conjugate (Ab-AuNP), the Ab was conjugated to p-isothiocyanatobenzyl-desferrioxamine (p-SCN-DFO) and subsequently conjugated to NHS-activated gold nanoparticles. The Ab-AuNP was characterized by transmission electron microscopy (TEM) and atomic force microscopy (AFM). Modified Lindmo assay was performed to assess binding affinity and internalization potential in vitro. The Ab-AuNP was radiolabeled with 89Zr and injected into CA19.9-positive BxPc-3 pancreatic orthotopic tumor-bearing mice pretreated with or without clodronate liposomes for PET imaging and biodistribution studies. Inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis was used to confirm delivery of gold nanoparticles to BxPc-3 pancreatic subcutaneous xenografts. [1] Subcutaneous tumor model:[1] Female athymic nude mice were injected with 5x106 BxPC-3 or MiaPaCa-2 cells in 150 μL (1:1 cell media:matrigel) in the hind flank. Tumors were allowed to grow for 3–4 weeks before imaging and biodistribution studies. Orthotopic tumor model:[1] For orthotopic pancreatic xenografts, female athymic nude mice were used. Mice were anesthetized with 1–2% isoflurane and surgeries were conducted on a heated pad to regulate body temperature during the procedure. Bupivicaine was administered as a local anesthetic intradermally in the area near the incision. Skin around the incision was washed with 3 alternating wipes of povidone-iodine and 70% ethanol. An incision through the skin and peritoneum was made, followed by removal of the spleen and pancreas from the peritoneal cavity. At this time, 6 × 105 BxPC-3 (luciferase-transfected) cells in media and Matrigel (1:1 ratio) were injected into the head of the pancreas. The spleen and pancreas were inserted back into the peritoneal cavity, which was then sutured with 4-0 Vicryl sutures. To close the skin, three sterile wound clips were inserted. Buprenorphine and meloxicam were administered immediately following the surgery. Meloxicam was administered 24 and 48 h post-surgery and wound clips were removed 7 days later. Tumor growth was monitored by bioluminescent imaging (IVIS Spectrum) with tumors reaching optimal size in approximately three weeks. Clodronate liposomes were obtained from Formumax and each mouse received a 200 μL intraperitoneal injection. ICP-OES analysis:[1] Analysis was carried out using an Optima 7000 DV spectrometer. The harvested organs containing [89Zr]Zr-5B1-AuNP and [89Zr]Zr-IgG-AuNP were first digested with an aqua regia solution of HNO3(65%): HCl (35%) at 75°C overnight and then dissolved in adequate volumes of 5% HNO3 solutions to be within the calibration curve range (from ppm to ppb). Hydrogen peroxide was added to speed the digestion of the organic materials. Calibration solutions were prepared from certified stock of a gold single element solution. The instrument was calibrated using a six-point calibration curve between 0.01 and 5 ppm and checked by three QC samples at the low, middle and high points on the curve. The operating conditions employed for ICP-OES determination were 1,300 W RF power, 15 L.min−1 plasma flow, 0.5 L.min−1 auxiliary flow, 0.8 L.min−1 nebulizer flow, and 1 mL.min−1 sample uptake rate. Signals at a wavelength of 267.595 nm were monitored. The low limit of quantification was determined to be 0.06 ppm. The comparison between the radioactive biodistribution data and ICP data is meant to be qualitative, in that it shows the same pattern of uptake. The discrepancy noted here we believe is due to the process by which the ICP measurements happen. Depending on the volume of acid required to dissolve organs, some concentrations may be below the limit of detection of the instrument (please note that the error bars are quite large for the ICP data due to this fact). This is a limitation of this method when using whole organs, as the volume of acid required for complete dissolution can be quite high; as well, a low injected mass of NPs, because it is difficult to dissolve, can make detection difficult for some samples, especially from the liver. Biodistribution studies:[1] Biodistribution studies were conducted by sacrificing mice at discrete time points after injection of antibody-nanoparticle conjugates. Relevant organs and tumors were harvested, weighed, and counted on a gamma counter. Biodistribution values are presented as the percent of the injected dose per gram of tissue and were calculated by including appropriate standards. PET/CT Imaging:[1] Mice were anesthetized with 1–2% isoflurane and images were acquired on an Inveon microPET/CT instrument. |
| 参考文献 | |
| 其他信息 |
In this work we have demonstrated the ability to deliver specifically targeted antibody-labeled gold nanoparticles to both subcutaneous and orthotopic pancreatic xenografts. When utilizing the humanized antibody 5B1, gold nanoparticles accumulated in subcutaneous pancreatic xenografts bearing the target antigen at 24.0 ± 11.6% ID/g compared to 4.0 ± 1.2% ID/g for the IgG-labeled control. This accounts for a 6–8x increase in tumors that expressed the target antigen. In an orthotopic model, 5B1-labeled gold nanoparticles accumulated 4–7 times more in tumors than did the IgG-labeled controls. Further, the ability of clodronate liposomes to enhance imaging of orthotopic pancreatic xenografts has also been demonstrated. This work carries implications for the development of methods of tracking nanoparticle biodistribution in real time in vivo. By incorporating a PET-active radionuclide onto our nanoparticles, we could visualize and analyze the effects of an active targeting moiety and macrophage depletion strategies. This could in theory then be used for other modifications made to any nanoparticle system that needs to be evaluated in vivo for clinical applications. The incorporation of a PET nuclide can enable the noninvasive tracking of drug effects or other factors in the biodistribution of nanoformulations. Further, gold nanoparticles themselves provide advantages such as (a) in vivo safety, (b) easy modification, and (c) the ability to target both passively through localization to the tumor microenvironment and actively by tumor cells. Lastly, this study supports a pharmacologic strategy that uses clodronate liposomes to enhance the biodistribution of a gold nanoparticle-antibody conjugate in order to enable imaging by PET. [1]
|
| 分子式 |
C33H52N8O8S2
|
|---|---|
| 分子量 |
752.94
|
| 精确质量 |
752.334
|
| CAS号 |
1222468-90-7
|
| PubChem CID |
24983484
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
1.5
|
| tPSA |
280
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
26
|
| 重原子数目 |
51
|
| 分子复杂度/Complexity |
1140
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HBAYEVATSBINBX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C33H52N8O8S2/c1-26(42)39(47)22-8-2-5-19-34-29(43)15-17-31(45)40(48)23-9-3-6-20-35-30(44)16-18-32(46)41(49)24-10-4-7-21-36-33(51)38-28-13-11-27(12-14-28)37-25-50/h11-14,47-49H,2-10,15-24H2,1H3,(H,34,43)(H,35,44)(H2,36,38,51)
|
| 化学名 |
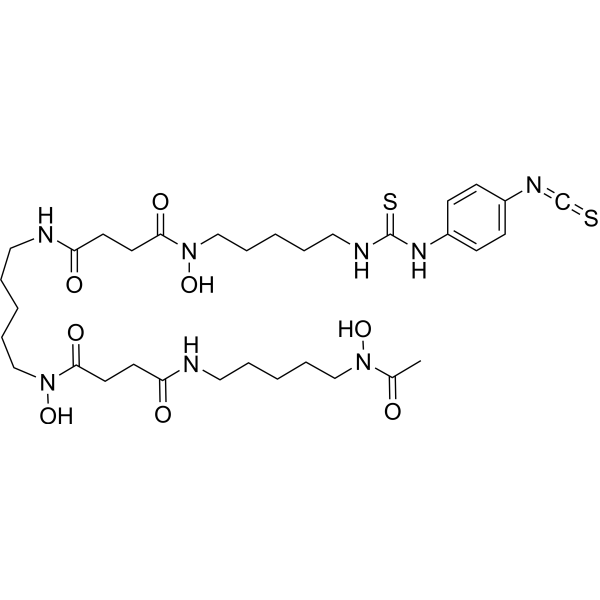
N-[5-[acetyl(hydroxy)amino]pentyl]-N'-hydroxy-N'-[5-[[4-[hydroxy-[5-[(4-isothiocyanatophenyl)carbamothioylamino]pentyl]amino]-4-oxobutanoyl]amino]pentyl]butanediamide
|
| 别名 |
p-SCN-Bn-deferoxamine; Berdoxam; 1222468-90-7; p-NCS-Bz-DFO; Berdoxam [USAN]; TMK6ND3QJH; UNII-TMK6ND3QJH; p-Isothiocyanatobenzyl-desferrioxamine;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3281 mL | 6.6406 mL | 13.2813 mL | |
| 5 mM | 0.2656 mL | 1.3281 mL | 2.6563 mL | |
| 10 mM | 0.1328 mL | 0.6641 mL | 1.3281 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。