| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
Muscle relaxant.
|
|---|---|
| 体外研究 (In Vitro) |
在离体犬心肌中,metubine (>15.0×10-3 g/L) 会导致等长力 (F) 和最大力发展速度 (dF/dt) 呈剂量依赖性下降[1]。
|
| 体内研究 (In Vivo) |
用离体犬心肌制剂研究了五种非去极化肌肉松弛剂的肌力增强作用。除法唑安定外,所有药物均以市售形式进行了研究。d-管库林氯(dTc)和metocurine iodide/碘化甲托库林(MTC)在浓度大于22.5 × 10(-3) g/L和大于15.0 × 10(-3) g/L时,会产生等长力(F)和最大力发展速度(dF/dt)的剂量依赖性降低,浓度分别比临床估计血清浓度高3倍和6倍。在同等浓度下,MTC组的心肌抑制比dTc组少约3倍。MTC对心肌F和dF/dt的抑制程度与MTC的防腐剂苯酚几乎相同,表明MTC引起的心肌抑制可能是由于防腐剂的作用。泮库溴铵(PC)使F和dF/dt呈剂量依赖性增加,并使力达到峰值的时间缩短。普萘洛尔10(-6)m可抑制PC诱导的F、dF/dt和力峰值时间的变化。结果表明,PC具有β -肾上腺素能刺激介导的正性肌力作用。在5.0 × 10(-3)到60.0 × 10(-3) g/L的浓度范围内,氯化铝没有改变F或dF/dt。在低浓度(1.875 × 10(-2) g/L)条件下,溴化Frazadinium使F和dF/dt略有增加,但浓度的进一步增加使F和dF/dt恢复到对照水平。服用临床有效剂量为0.3 mg/kg的dTc、0.1 mg/kg的MTC或PC、0.2 mg/kg的氯化铝或0.75 mg/kg的溴化法唑铵的患者,体内血浆中的松弛剂浓度在体外不会改变F和dF/dt。[1]
|
| 药代性质 (ADME/PK) |
Biological Half-Life
3 to 4 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
35% in plasma man TDLo intravenous 150 ug/kg SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE; VASCULAR: BP LOWERING NOT CHARACTERIZED IN AUTONOMIC SECTION; LUNGS, THORAX, OR RESPIRATION: DYSPNEA Journal of Pharmacology and Experimental Therapeutics., 93(109), 1948 rat LD50 intraperitoneal 370 ug/kg PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE); LUNGS, THORAX, OR RESPIRATION: DYSPNEA Journal of Pharmacology and Experimental Therapeutics., 93(109), 1948 rat LD50 intravenous 35 ug/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES Journal of Laboratory and Clinical Medicine., 34(516), 1949 mouse LD50 intravenous 230 ug/kg BEHAVIORAL: MUSCLE WEAKNESS Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie., 244(493), 1963 [PMID:13987623] rabbit LD50 intravenous 32 ug/kg BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES Journal of Laboratory and Clinical Medicine., 34(516), 1949 |
| 参考文献 |
[1]. Iwatsuki N, et al. Inotropic effects of non-depolarizing muscle relaxants in isolated canine heart muscle. Anesth Analg. 1980 Oct;59(10):717-21.
[2]. Durant NN, et al. A comparison of the neuromuscular and autonomic blocking activities of (+)-tubocurarine and its N-methyl and O,O,N-trimethyl analogues. Eur J Pharmacol. 1977 Dec 15;46(4):297-302. |
| 其他信息 |
Metocurine iodide is an aromatic ether.
Metocurine iodide is a benzylisoquinolinium competitive nondepolarizing neuromuscular blocking agent. It is used as an anesthesia adjunct to induce skeletal muscle relaxation and to reduce the intensity of muscle contractions in convulsive therapy Metocurine iodide has a moderate risk of inducing histamine release and has some ganglion blocking activity. Metocurine iodide can be used most advantageously if muscle twitch response to peripheral nerve stimulation is monitored to assess degree of muscle relaxation. Metocurine Iodide is no longer available on the US market. See also: Metocurine (has active moiety). Drug Indication For use as an anesthesia adjunct to induce skeletal muscle relaxation and to reduce the intensity of muscle contractions in convulsive therapy. Mechanism of Action Metocurine iodide antagonizes the neurotransmitter action of acetylcholine by binding competitively with cholinergic receptor sites on the motor end-plate. This antagonism is inhibited, and neuromuscular block reversed, by acetylcholinesterase inhibitors such as neostigmine, edrophonium, and pyridostigmine. Pharmacodynamics Metocurine iodide is a benzylisoquinolinium competitive nondepolarizing neuromuscular blocking agent. Metocurine iodide has a moderate risk of inducing histamine release and has some ganglion blocking activity. Metocurine iodide can be used most advantageously if muscle twitch response to peripheral nerve stimulation is monitored to assess degree of muscle relaxation. As with other nondepolarizing neuromuscular blockers, the time to onset of paralysis decreases and the duration of maximum effect increases with increasing doses of metocurine iodide. Repeated administration of maintenance doses of metocurine iodide has no cumulative effect on the duration of neuromuscular block if recovery is allowed to begin prior to repeat dosing. Moreover, the time needed to recover from repeat doses does not change with additional doses. Repeat doses can therefore be administered at relatively regular intervals with predictable results. |
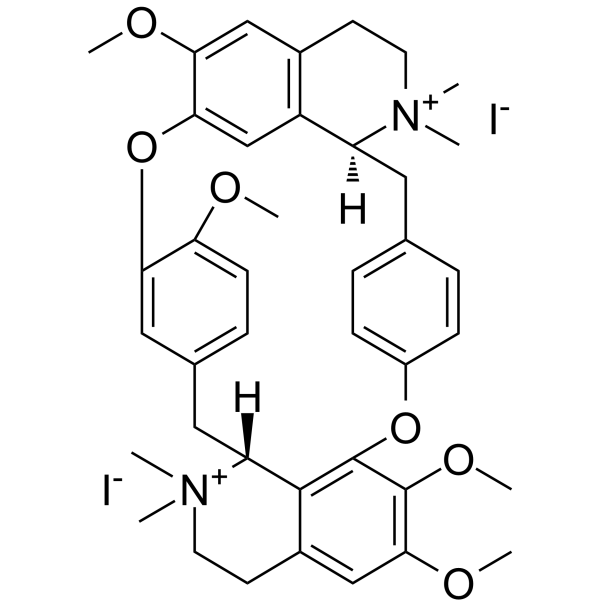
| 分子式 |
C40H48I2N2O6
|
|---|---|
| 分子量 |
906.63
|
| 精确质量 |
906.16
|
| CAS号 |
7601-55-0
|
| 相关CAS号 |
5152-30-7 (Parent)
|
| PubChem CID |
24244
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 熔点 |
267-270
|
| LogP |
1.377
|
| tPSA |
55.38
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
50
|
| 分子复杂度/Complexity |
1060
|
| 定义原子立体中心数目 |
2
|
| SMILES |
[I-].[I-].COc1ccc2CC3c4c(CC[N+]3(C)C)cc(OC)c(OC)c4Oc3ccc(CC4c5cc(Oc1c2)c(OC)cc5CC[N+]4(C)C)cc3
|
| InChi Key |
DIGFQJFCDPKEPF-OIUSMDOTSA-L
|
| InChi Code |
InChI=1S/C40H48N2O6.2HI/c1-41(2)17-15-27-22-34(44-6)36-24-30(27)31(41)19-25-9-12-29(13-10-25)47-40-38-28(23-37(45-7)39(40)46-8)16-18-42(3,4)32(38)20-26-11-14-33(43-5)35(21-26)48-36;;/h9-14,21-24,31-32H,15-20H2,1-8H3;2*1H/q+2;;/p-2/t31-,32+;;/m0../s1
|
| 化学名 |
(1S,16R)-9,10,21,25-tetramethoxy-15,15,30,30-tetramethyl-7,23-dioxa-15,30-diazoniaheptacyclo[22.6.2.23,6.18,12.118,22.027,31.016,34]hexatriaconta-3(36),4,6(35),8(34),9,11,18(33),19,21,24,26,31-dodecaene;diiodide
|
| 别名 |
METOCURINE IODIDE; Metubine iodide; 7601-55-0; Dimethylchondrocurarine iodide; Methyl-curarin; Tetrandrini dimethiodidum; Dimethyl tubocurarine iodide; O,O'-Dimethylchondrocurarine diiodide;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1030 mL | 5.5149 mL | 11.0299 mL | |
| 5 mM | 0.2206 mL | 1.1030 mL | 2.2060 mL | |
| 10 mM | 0.1103 mL | 0.5515 mL | 1.1030 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。