| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Cyclic Adenosine monophosphate (cAMP)[5]
|
|---|---|
| 体外研究 (In Vitro) |
人肺上皮细胞A549的活力不受千日菊素(50~150 μM,24h)的影响[3]。以 50~150 μM 浓度作用 24 小时,千日菊素对人肺上皮细胞系 A549 具有抗炎特性 [3]。当使用千日菊素 (50–150 μM) 24 小时时,ICAM-1 基因在 A549 人肺上皮细胞系中的表达量会减少 [3]。
Spilanthol是一种从Spilanthes acmella植物中提取的植物化学物质,具有抗菌、抗氧化和抗炎的特性。本研究评估了其对il -1β刺激的人肺上皮A549细胞细胞间粘附分子1 (ICAM-1)和炎症相关介质表达的影响。采用不同浓度(3 ~ 100 μM)的spilanthol预处理人肺上皮A549细胞,再用IL-1β诱导炎症反应。采用ELISA法检测促炎细胞因子、趋化因子和前列腺素E2 (PGE2)蛋白水平。免疫印迹法检测环氧化酶-2 (COX-2)、血红素加氧酶(HO-1)、核转录因子κ b (NF-κB)、丝裂原活化蛋白激酶(MAPK)水平。实时聚合酶链反应检测ICAM-1和MUC5AC mRNA表达水平。Spilanthol降低PGE2、COX-2、TNF-α和MCP-1的表达。它还能降低ICAM-1的表达,抑制单核细胞对il -1β刺激的A549细胞的粘附。Spilanthol还能显著抑制MAPK和I-κB的磷酸化。这些结果表明,spilanthol通过抑制NF-κB和MAPK信号通路,抑制促炎细胞因子、COX-2和ICAM-1的表达,从而发挥抗炎作用。[3] |
| 体内研究 (In Vivo) |
在雄性 ICR 小鼠模型中,乙酸促进腹部扭曲,辣椒素导致舔爪,千日菊素(1-1.875 mg/kg,Ip,一次)发挥镇痛作用[2]。瑞士小鼠 5-氟尿嘧啶诱导的肠道粘膜炎模型显示,当给予千日菊素(30 mg/kg,口服,每天一次,持续 4 天)时,对肠道损伤具有保护作用[4]。成年雄性 C57BL/6J 小鼠易受千日菊素(800 mg/kg,口服,一次)的利尿作用影响[5]。
粘膜炎是癌症患者接受放疗和/或化疗时最常见的副作用之一,目前缺乏适当有效的治疗方法。马齿苋是一种来自南美洲的开花草本植物,含有spilanthol,一种具有多种药理特性的烷基酰胺,包括麻醉、镇痛和抗炎活性。因此,这项工作的目的是评估spilanthol对5-氟尿嘧啶(5-FU)引起的瑞士小鼠肠黏膜炎的影响,5-氟尿嘧啶是一种用于治疗多种不同癌症的抗肿瘤药物。反复给药5-FU导致所有动物出现肠黏膜炎,并随之减少食物摄入量,同时体重减轻。与仅5-FU组相比,每日给药30 mg/kg施泼酚显著降低了肠黏膜炎的严重程度,减少了组织病理学变化,增加了绒毛高度(p < 0.0044)。在30 mg/kg施泼酚处理的动物中也观察到髓过氧化物酶活性的降低(p < 0.05),尽管在任何组中都无法量化几种促炎细胞因子。综上所述,这些数据表明,在5-FU诱导的小鼠肠黏膜炎模型中,spilanthol有效地减轻了炎症,该化合物可能是一种有希望的预防和治疗这种疾病的候选药物。[4] 在巴西传统医学中,马蹄莲被公认为利尿剂,尽管很少有科学数据发表来支持这种效果。本研究的目的是研究花青树提取物及其主要的烷基酰胺spilanthol对尿浓缩机制中两个主要过程的影响:Na-K-2Cl同向转运体(NKCC2)在粗升肢中的活性和水通道水通道蛋白2在收集管细胞顶质膜上的积累。Western blotting检测NKCC2的磷酸化水平作为其活化的指标。用激光共聚焦显微镜分析水通道蛋白2的顶端表达率。通过视频成像实验解剖了spilanthol诱导的细胞内信号事件。暴露于spilanthol降低了新分离的小鼠肾片和表达NKCC2的HEK293细胞中NKCC2的基础磷酸化水平。此外,在小鼠肾切片和表达NKCC2的HEK293细胞中,暴露于spilanthol分别强烈降低了去氨加压素和低Cl依赖性NKCC2磷酸化的增加。同样,spilanthol也减少了去氨加压素和forskolin刺激的小鼠肾片和MCD4细胞收集管顶端质膜水通道蛋白2的积累。值得注意的是,与对照组小鼠相比,经口服给药后,泼茶酚诱导尿量和尿盐排泄量显著增加,并显著降低尿渗透压。最后,在细胞水平上,通过细胞内[Ca2+]增加的机制,spilanthol迅速降低或逆转基础和激动剂增加的cAMP水平。总之,spilanolol诱导的cAMP产生抑制负调节尿浓缩机制,因此它作为利尿剂的应用前景广阔。[5] |
| 酶活实验 |
促炎因子、趋化因子和PGE2 [3]
的测定 A549细胞(106个/mL)在24孔板上用不同浓度的spilanthol (50-150 μ5)培养1 h,然后加入IL-1β (1 ng/mL),再培养24 h,上清液采用MCP-1、TNF-α、PGE2和ICAM-1特异性ELISA试剂盒检测。在450 nm处用分光光度法测定外径。 |
| 细胞实验 |
细胞活力测定[3]
细胞类型: A549人肺上皮细胞系 测试浓度: 50μM、75μM、100μM、150 μM 孵育时间:24小时 实验结果:细胞活力没有明显变化。 细胞毒性测定[3] 细胞类型: A549人肺上皮细胞系 测试浓度: 50μM、75μM、100μM、 150 μM 孵育时间: 24h 实验结果: 显着抑制炎症细胞因子 TNF-α 和趋化因子 MCP-1 的释放。 蛋白质印迹分析[3] 细胞类型: A549人肺上皮细胞系 测试浓度: 50μM、75μM、100μM、 150 μM 孵育时间: 24h 实验结果: 抑制COX-2表达并增加HO-1表达。 细胞活力测定[3] 细胞类型: A549人肺上皮细胞系 测试浓度: 50μM、75μM、100μM、 150 μM 孵育时间: 24 小时 实验结果: THP-1 细胞粘附显着减少。 |
| 动物实验 |
Animal/Disease Models: Acetic acid-induced abdominal writhes Male ICR mice model[2]
Doses: 1-1.875 mg/kg Route of Administration: intraperitoneal (ip) administration(ip), Once Experimental Results: As the dose increased, the number of abdominal contractions and licking behavior diminished, and a maximum antinociceptive effect of 46.67%. Animal/Disease Models: Swiss mice model of intestinal mucositis induced by 5-fluorouracil[4]. Doses: 30 mg/kg Route of Administration: Oral adminstraion(po), one time/day for four days Experimental Results: demonstrated the Intestinal wall recovery, villi are higher and less irregularity and high number and greater length of intestinal crypts. Animal/Disease Models: Adult C57BL/6J male mice[5]. Doses: 800 mg/Kg Route of Administration: Oral adminstraion (po), Once Experimental Results: Increased in urine output, sodium, and increased excretion of sodium, potassium and chloride. |
| 毒性/毒理 (Toxicokinetics/TK) |
mouse LD50 oral 4378 mg/kg BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX); BEHAVIORAL: TREMOR Toho Igakkai Zasshi. Journal of Medical Society of Toho University., 17(277), 1970
|
| 参考文献 |
|
| 其他信息 |
Spilanthol has been reported in Acmella oleracea and Heliopsis longipes with data available.
Spilanthol (C14H23NO, 221.339 g/mol) (1) is a bioactive compound that is found in many different plants that are used as traditional remedies throughout the world (Molinatorres et al., 1996, Prachayasittukal et al., 2013, Paulraj et al., 2013, Rios and Olivo, 2014). Its IUPAC name is (2E,6Z,8E)-N-isobutyl-2,6,8-decatrienamide (Molinatorres et al., 1996). It is also known as affinin. Spilanthol has many biological activities (Dubey et al., 2013), including analgesic, antinociceptive, anti-inflammatory, antimutagenic, anti-wrinkle, antifungal, bacteriostatic, insecticidal, anti-malarial, anti-larvicidal activities against Aedes aegypti and Helicoverpa zea neonates, and anti-molluscicidal activities (Johns et al., 1982). There have also been reports on its activities as an anticonvulsant, antioxidant, aphrodisiac, pancreatic lipase inhibitor, antimicrobial agent, antinociceptive agent, diuretic, vasorelaxant, anti-human immunodeficiency virus, toothache relief and as an anti-inflammatory agent (Dubey et al., 2013). It can be absorbed through the skin, endothelial gut, oral mucosa and blood–brain barrier. It can enhance the ability of caffeine, fortestosterone and five mycotoxins to penetrate the skin (De Spiegeleer et al., 2013). So, it is important to make sure that formulations containing spilanthol are not contaminated with mycotoxins (De Spiegeleer et al., 2013). It also improved male sexual performance in rats as indicated by penile erection, mounting frequency, intromission frequency, ejaculation frequency that lasted even 14 days after discontinuing its administration. The antinociceptive activity of spilanthol was studied in detail (Déciga-Campos et al., 2010). Intraperitoneal administration of 30 mg/kg spilanthol produced an antinociceptive dependent-dose effect when assessed in mice submitted to acetic acid and capsaicin tests. Spilanthol-induced antinociception was blocked by naltrexone, p-chlorophenylalanine and flumazenil. So, its antinociceptive effect may be due to the activation of opiodergic, serotoninergic and GABAergic systems. Moreover, the antinociceptive effect decreased when mice were pretreated with 1H-[1,2,4]oxadiazolo[1,2-a]quinoxalin-1-one and glibenclamide. This supports the idea that the nitric oxide-K+ channels pathway could be involved in the mechanism of action (Déciga-Campos et al., 2010). Subsequently, the same group found that spilanthol not only had a antinociceptive effect, but it also modified anxiety behavior and prolonged the time of sodium pentobarbital-induced hypnosis. They also found that spilanthol decreased the time of clonic and tonic seizures that were induced by pentylenetetrazole (PTZ). Analgesic activity was studied by evaluating the inhibition of acetic acid induced writhing in mice (Ogura et al., 1982). Spilanthol was administered orally in aqueous solutions at doses ranging from 2.5 to 10.0 mg/kg. It exhibited an ED50 of 6.98 mg/kg. The analgesic activity of spilanthol was attributed to increased GABA release in the temporal cerebral cortex (Ogura et al., 1982). In another study, spilanthol caused GABA to be released 0.5 min after being administered at a concentration of 1 × 10−4 M. One other study found that spilanthol displayed analgesic action similar to ketorolac (Cilia-López et al., 2010). Also, its stimulating effect on the nervous system of adult mice was comparable to caffeine. The antimutagenic activity of spilanthol was demonstrated by its ability to reduce 2AA- and NOR-induced mutations inTA98 and TA102 strains of Salmonella Typhimurium (Arriaga-Alba et al., 2013). Spilanthol (25 and 50 μg/plate) significantly reduced the frameshift mutations that were generated by 2-aminoanthracene (2AA) (40%) and reduced the oxidative DNA damage generated by norfloxacin (NOR) (37–50%). The antioxidant power of spilanthol and extracts of A. oleracea have also been studied (Abeysiri et al., 2013). One study found 5.29, 1.42 and 3.42 mg of trolox equivalents per g of dry leaves, stems and flowers (Abeysiri et al., 2013). It also found 7.59, 1.65 and 5.34 mg of gallic acid equivalents per gram dry weight (mg GAE/g DW) of total phenolic compounds (Abeysiri et al., 2013). A different study found 9.2, 10.3 and 7.7 mg of trolox equivalents per g of dry arial parts of A. oleracea grown three different ways: in the field, with hydroponics and as a callus, respectively (Abeysinghe et al., 2014). The same study found 11.0, 11.5 and 9.9 mg GAE/g DW total phenolics in A. oleracea grown in the field, with hydroponics and as a callus, respectively (Abeysinghe et al., 2014). The total flavonoid content was 11.3, 12.3 and 7.4 mg rutin equivalents per gram of dry weight in A. oleracea grown in the field, with hydroponics and as a callus, respectively. The anti-inflammatory activity of dried flowers was demonstrated on the commonly used lipopolysaccharide-activated murine macrophage model, RAW 264.7 (Wu et al., 2008). These macrophages produce nitric oxide (NO) to mediate inflammation, through an inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Spilanthol inhibited the production of iNOS and COX-2 and the mRNA that code for them. It was also suggested that spilanthol attenuates the inflammatory responses in murine RAW 264.7 macrophages partly due to the inactivation of NF-κB. This down regulates the production of proinflammatory mediators. Spilanthol also had an anti-inflammatory effect on the arachidonic acid model with ED50 = 1.2 mg/ear (Wu et al., 2008). In a different study using the phorbol myristate acetate model, spilanthol showed an anti-inflammatory dose-dependent effect with ED50 = 1.3 mg/ear (Hernández et al., 2009). Extracts containing spilanthol have been used to treat toothaches, stomatitis and skin diseases such as swimmer's eczema (Boonen et al., 2010a, Boonen et al., 2010b). Extracts and spilanthol are in buccal mucosa preparations that are indicated for a painful mouth and minor mouth ulcers. Several spilanthol containing preparations for buccal use are commercially available (Boonen et al., 2010a, Boonen et al., 2010b). Also, spilanthol has been incorporated in tooth pastes and mouth rinses. The objective is to provide a lasting fresh minty flavor; it also increases salivation, which improves appetite. The spilanthol present also has a mild anesthetic effect thus enabling people with toothache to brush comfortably (Hatasa and Iioka, 1973). There is also a patent for manufacturing toothpastes or other oral compositions with spilanthol-rich essential oils (Shimada and Gomi, 1995). A mouthwash contained ethanol 10.0, 85% glycerin 8.0, 65% sorbitol 2.0, chlorohexidine gluconate 0.05, triclosan 0.003, menthol 0.01, peppermint oil 0.01, sodium saccharin 0.001, spilanthol-rich essential oil 0.01 wt.% and balance purified water. Also, spilanthol in A. oleracea L. extracts inhibited contractions in subcutaneous muscles, notably those of the face, and can be used as an anti-wrinkle product (Demarne and Passaro, 2009). As a result, many anti-aging products containing spilanthol such as Gatuline®, SYN®-COLL and ChroNOline™ are available. The antifungal and bacteriostatic activities of spilanthol and other alkamides from the roots of H. longipes were also studied (Molina-Torres et al., 2004). Four of the assayed fungi showed growth inhibition of 100% due to the presence of spilanthol: Sclerotium rolfsii, S. cepivorum, Phytophthora infestans, and Rhizoctonia solani AG-3 and AG-5. Spilanthol also inhibited the growth of Bacillus subtilis, Escherichia coli and Saccharomyces cerevisiae at concentrations as low as 25 μg/ml (Molina-Torres et al., 2004). In another study, spilanthol in S. calva was found to have antifungal activity against the fungi Fusarium oxysporum and Trichophyton mentagrophytes (Rai et al., 2004). This antifungal activity was enhanced when S. calva was inoculated with the root endophyte Piriformospora indica, which also increased the concentration of spilanthol in the roots of S. calva. Spilanthol was also shown to be useful as an insecticide (Kadir et al., 1989, Spelman et al., 2011, Sharma et al., 2012). It killed the diamondback moth, Plutella xylostella L, which is one of the most destructive pests that attack cruciferous vegetables, such as broccoli (Sharma et al., 2012). Spilanthol was also able to kill the tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), which attacks solanaceous plants and has become a serious threat to tomatoes in the Mediterranean region (Moreno et al., 2012). Electrophysiological studies indicated immediate hyperexcitation followed by complete inhibition of the cockroach cercal nerve activity. Spilanthol exhibited the highest toxicity to Tuta absoluta, with the lowest LD50 (0.13 μg mg−1). Furthermore, spilanthol was approximately five times more toxic than permethrin and approximately 321 times more potent than Azadirachta indica extract. On the other hand, spilanthol was not toxic to two beneficial insects, the predator Solenopsis saevissima (Smith) (Hymenoptera: Formicidae) and the pollinator, tetragonisca angustula (Latr.) (Hymenoptera: Apidae: Melipninae) (Moreno et al., 2012). Even more important, spilanthol has been shown to be toxic to the mosquitoes (Plasmodium falciparum) that carry malaria (Spelman et al., 2011). It had an IC50 of 16.5 μg/ml and 41.4 μg/ml on P. falciparum strain PFB and IC50 of 5.8 μg/ml and 16.3 μg/ml for the chloroquine resistant P. falciparum K1 strain, respectively. Further investigations revealed that at relatively low concentrations, spilanthol and the water extract of S. acmella reduced the parasitemia 59 and 53% in mice infected with P. yoelii yoelii 17XNL at 5 and 50 mg/kg, respectively. This parasite is used to infect mice in an animal model of malaria. These results provide evidence supporting the antimalarial activities of S. acmella and spilanthol (Spelman et al., 2011). Finally, another group reported the ability of extracts of S. acmella Murr. to kill the American cockroach, Periplaneta americana L. (Kadir et al., 1989). The potency was found to be 1.3, 2.6 and 3.8 times more toxic than carbaryl, bioresmethrin and lindane, respectively . Spilanthol is also active against Aedes aegyptii larvae, which can spread the viruses that cause dengue fever, chikungunya, and yellow fever as well as Helicoverpa zea neonates (corn earworm) at concentrations of 12.5 and 250 mg/ml, respectively (Ramsewak et al., 1999). Spilanthol, at 7.5 ppm concentration, caused 100% motility of eggs, larvae, and pupae of Anopheles, Culex, and Aedes mosquitoes at lower doses; it is also effective against eggs and pupae (Saraf and Dixit, 2002). The insecticidal activity of Heliopsis longipes roots against Anopheles albimanus and Aedes aegypti was determined (Hernández-Morales et al., 2015). A concentration of 7 mg/l of ethanolic extract caused 100% of larval mortality for A. albimanus, and had the same effect on A. aegypti larvae. This effect could be attributed to spilanthol. The conjugated double bonds present in its structure were found to be necessary to maintain larvicidal activity. This study demonstrated the potential of H. longipes for controlling the larval stage of A. albimanus and A. aegypti, transmitter vectors of malaria and dengue fever, respectively. Others explored Spilanthes acmella Murr. for insecticidal activity (Sharma et al., 2012). The seed extract and spilanthol were toxic to Plutella xylostella. An activity of 95–100% was observed at a dose of 2 g/l of spilanthol, while 60–70 and 80–90% mortality was seen in crude seed extracts prepared in methanol and hexane at a dose of 5 g/l after 48 h exposure. LC50 values of 1.49, 5.14, 5.04, 11.75 g/l were observed for spilanthol, crude methanolic seed extract, hexane extracts and deltamethrin, respectively. These findings indicated the potential of S. acmella and spilanthol for controlling P. xylostella and other insects of agricultural importance (Sharma et al., 2012). Spilanthol also has strong molluscicidal activity against Physa occidentalis (LD50 of 100 μM) and the cercariae of the fluke (Johns et al., 1982). At a concentration of 50 mg/l in water at 21° snails were inactive after 60 min and dead within 18 h. At 150 mg/l (the solubility limit for spilanthol) cercarial emergence ceased and the snails showed immobility after 30 min. Cercariae ceased to move after five set and convulsed after 1 min. Spilanthol also can also stimulate the growth of roots in Arabidopsis thaliana seedlings (Campos-Cuevas et al., 2008). Although the effects of spilanthol was similar to those produced by auxins on adventitious root development, the ability of shoot explants to respond to spilanthol was found to be independent of auxin signaling. These results suggest a role for spilanthol in regulating adventitious root development, probably operating through the NO signal transduction pathway. Spilanthol was also shown to inhibit CYP P450 enzymes, with IC50 values of 25, 16.1 and 13.5 μg/ml for CYP1A1/2, CYP2D6 and CYP3A4, respectively (Rodeiro et al., 2009). These results suggest that spilanthol inhibits the major human P450 enzymes involved in drug metabolism and could induce potential herbal–drug interactions (Smith, 2014). On the other hand, CYP1A1/2 inhibition could be associated with decreased carcinogenic risk. Although, in vitro inhibition of P450s does not necessarily lead to relevant in vivo effects, these results recommend a cautious evaluation of the potential clinical consequences derived from the consumption of these products, particularly for long-term treatments . In conclusion, spilanthol is a secondary metabolite with high industrial potential as well as several biological properties and health effects. It can be found, extracted and purified from A. oleracea and H. longipes. A. oleracea is used as a spice and a food in the northern part of Brazil. It is also used as a treatment for treating toothaches, so it is called the toothache plant. Spilanthol may also have analgesic, antinociceptive, antioxidant, anti-inflammatory, antimutagenic (Arriaga-Alba et al., 2013), anti-wrinkle (Demarne and Passaro, 2009), antifungal (Dubey et al., 2013), bacteriostatic (Molina-Torres et al., 2004), insecticidal (Kadir et al., 1989, Sharma et al., 2012, Moreno et al., 2012), anti-malarial (Soares et al., 2014), anti-larvicidal against Aedes aegypti and Helicoverpa zea neonates (Ramsewak et al., 1999), and anti-molluscicidal (Johns et al., 1982). There have also been reports on its activities as an anticonvulsant, antioxidant, aphrodisiac, pancreatic lipase inhibitor, antimicrobial agent, antinociceptive agent, diuretic, vasorelaxant, anti-human immunodeficiency virus, toothache relief and anti-inflammatory (Dubey et al., 2013). The biological activities are listed in Box 1. [1] To summarize, we found that spilanthol not only inhibited the levels of TNF-α and MCP-1, but it also suppressed COX-2 protein expression and promoted HO-1 protein expression by suppressing NF-κB activation and MAPK pathways in IL-1β-activated human lung epithelial cells. In addition, we found evidence that spilanthol decreased ICAM-1 expression in these cells. Based on these results, we propose a model that explains the anti-inflammatory effects of spilanthol. We conclude that spilanthol, which is a natural anti-inflammatory agent, acts as a regulatory factor in MAPK pathways and in NF-κB activation of COX-2 and ICAM-1 expression. Further studies are needed to investigate its effects in vivo. [3] |
| 分子式 |
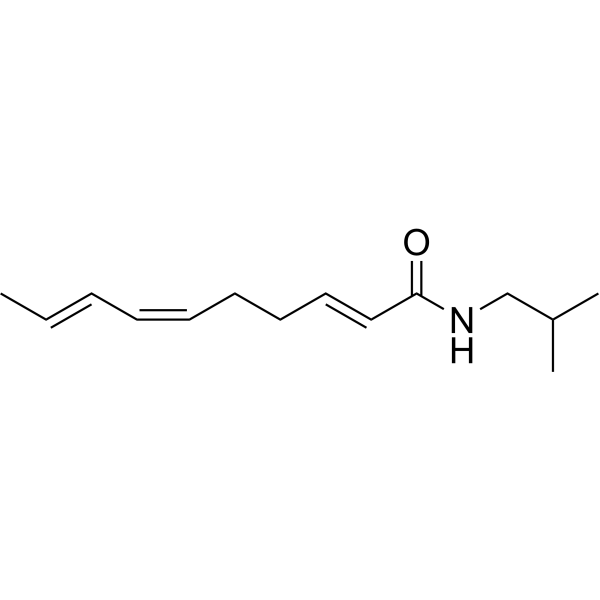
C14H23NO
|
|---|---|
| 精确质量 |
221.178
|
| CAS号 |
25394-57-4
|
| PubChem CID |
5353001
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| 蒸汽压 |
4.02E-06mmHg at 25°C
|
| LogP |
3.618
|
| tPSA |
29.1
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
262
|
| 定义原子立体中心数目 |
0
|
| SMILES |
C/C=C/C=C/CC/C=C/C(NCC(C)C)=O
|
| InChi Key |
BXOCHUWSGYYSFW-HVWOQQCMSA-N
|
| InChi Code |
InChI=1S/C14H23NO/c1-4-5-6-7-8-9-10-11-14(16)15-12-13(2)3/h4-7,10-11,13H,8-9,12H2,1-3H3,(H,15,16)/b5-4+,7-6-,11-10+
|
| 化学名 |
(2E,6Z,8E)-N-(2-methylpropyl)deca-2,6,8-trienamide
|
| 别名 |
Spilanthol; 25394-57-4; Affinin; (2E,6Z,8E)-N-(2-methylpropyl)deca-2,6,8-trienamide; N-Isobutyl-2(E),6(Z),8(E)-decatrienamide; (2E,6Z,8E)-N-Isobutyldeca-2,6,8-trienamide; 4W9L3S4856; 2,6,8-DECATRIENAMIDE, N-ISOBUTYL-, (E,E,Z)-;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
|---|
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。