| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| Other Sizes |
| 体外研究 (In Vitro) |
药物化合物包括碳、氢和其他元素的稳定重同位素,在药物开发过程中主要作为定量示踪剂。由于氘化可能会影响药物的药代动力学和代谢特性,因此值得关注[1]。
|
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Ursodiol is naturally present in human milk. Because of the low levels of ursodiol (ursodeoxycholic acid) in breastmilk after exogenous administration, amounts ingested by the infant are small and are not expected to cause any adverse effects in breastfed infants. Ursodiol has been given directly to newborns to safely and successfully treat prolonged neonatal jaundice. No special precautions are required. ◉ Effects in Breastfed Infants One breastfed (extent not stated) infant developed normally over the first 6 months of life during maternal ursodiol therapy of 750 to 1000 mg daily. Seven women who were taking ursodiol 14 mg/kg daily near term and postpartum. They reported no adverse reactions in their breastfed infants during the early postpartum period. A mother receiving oral ursodiol 250 mg 3 times daily for primary biliary cirrhosis reportedly breastfed her infant normally, although the extent and duration of breastfeeding was not stated. A woman with primary biliary cirrhosis developed severe pruritus and elevated serum bile acids 3 weeks postpartum. Ursodiol was started at a dose of 500 mg (7.5 mg/kg) daily, increasing to 1500 mg (25 mg/kg) daily over the next 8 weeks. Psychomotor development of her breastfed (extent not stated) infant was normal, and no apparent side effects were observed in the infant. A retrospective review of the medical records of pregnant patients at a hospital in Ankara, Türkiye who had a diagnosis of primary biliary cirrhosis found 8 patients who took ursodiol postpartum in doses of 13–15 mg/kg daily. “Most” of the patients breastfed their infants (extent not stated). No infant side effects were reported. A woman was breastfeeding her 8-day-old preterm infant 10 times daily for about 15 minutes each time. The infant was born by cesarean section at 34 weeks of gestation with a weight of 3600 grams. She was diagnosed with cholestasis, type 1 diabetes, and hypothyroidism. She was treated with ursodiol 500 mg daily, insulin levemir and aspart, and levothyroxine. She was also taking cefuroxime, flurbiprofen, a combination of acetaminophen, propyphenazone, and caffeine. The mother took the ursodiol for a total of 12 days, cefuroxime and the analgesic combination for 10 days and flurbiprofen for 15 days. No adverse effects were noticed during the period of ursodiol treatment. Twenty nursing mothers were taking ursodiol for cholestasis in daily dosages of 500 to 1500 mg or 13 to 15 mg/kg, depending on the condition. Ursodiol was discontinued 3 days postpartum. No apparent side effects were observed in any newborn infant based on standard clinical examination during early postnatal period, and no deterioration in postnatal development was observed during routine 1-year follow-up on routine pediatric examinations. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 | |
| 其他信息 |
3,7-Dihydroxycholan-24-oic acid has been reported in Anser anser with data available.
|
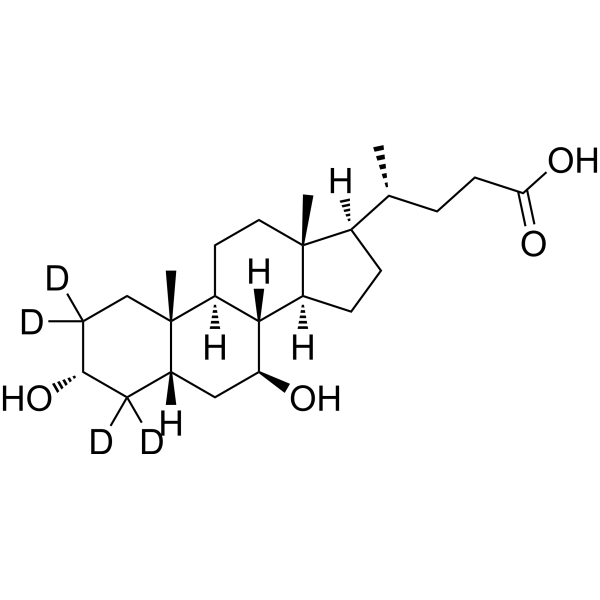
| 分子式 |
C24H36D4O4
|
|---|---|
| 分子量 |
396.60
|
| 精确质量 |
396.318
|
| CAS号 |
347841-46-7
|
| PubChem CID |
5645
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4.477
|
| tPSA |
77.76
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
605
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
RUDATBOHQWOJDD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)
|
| 化学名 |
4-(3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5214 mL | 12.6072 mL | 25.2143 mL | |
| 5 mM | 0.5043 mL | 2.5214 mL | 5.0429 mL | |
| 10 mM | 0.2521 mL | 1.2607 mL | 2.5214 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。