| 规格 | 价格 | |
|---|---|---|
| 500mg | ||
| 1g | ||
| Other Sizes |
| 靶点 |
Fluorescent Dye
|
|---|---|
| 体内研究 (In Vivo) |
可以使用 TMR Biocytin [2] 研究血脑屏障 (BBB) 通透性变化。指南(本协议仅作为指南;应对其进行调整以满足您的独特要求。以下是我们建议的协议)[2]。 1. 对于每只小鼠,将 1 毫克 TMR Biocytin 稀释在 100 μL PBS 中,然后将混合物注射到尾静脉中。 2.注射后半小时麻醉并灌注动物。 3. 取下脊髓,然后制作深度冷冻的连续 10 μm 纵向切片。 4. 使用 DAPI 进行核复染。 5. 对每个队列使用相同的激光强度、曝光时间和放大倍数,使用 ×10 物镜和 ×10 目镜拍摄整个切片的照片。 6. 使用来自注射示踪剂和未注射示踪剂的动物的肝脏样本来设定上述值。
TMR生物细胞素的初始纤维转运速度高达5.4mm/h。TMR生物胞素可以与AM钙染料结合使用,从几毫米的距离标记神经元胞体,并在几个小时内记录钙瞬变。TMR生物细胞蛋白的细胞外应用导致快速顺行运输,并在10分钟内标记局部突触。TMR生物细胞素是可固定的,在水杨酸甲酯清除过程中是稳定的,可以在神经组织深处观察到。 与现有方法相比:TMR生物细胞蛋白的逆行标记仅需几个小时即可实现远程神经元可视化和并发钙成像,这比其他基于荧光的示踪剂快得多。发绿光的Atto 488生物素也被吸收和逆向运输,但它与标准发绿光的钙染料不兼容。 结论:TMR生物细胞素是一种有吸引力的神经元示踪剂。它可以长距离快速标记神经元,并且可以与钙染料结合使用,以报告逆行标记的活神经元中的神经元活动。[1] 电休克疗法的脑刺激对神经精神疾病的有效机制尚不清楚。小胶质细胞毒性在神经精神、神经炎症和退行性疾病中起着关键作用。我们研究了电惊厥发作(ECS)调节小胶质细胞表型和对刺激反应的机制。通过形态学分析、Iba1和细胞因子表达检查小胶质细胞反应。ECS不影响静息小胶质细胞的表型或形态,但通过脂多糖刺激调节其活化。在幼年小鼠的ECS或假疗程后分离小胶质细胞进行转录组分析。RNA测序鉴定出141个差异表达基因。ECS调节多个免疫相关基因家族,并减弱神经毒性相关基因的表达。通过注射Biocytin TMR示踪剂检查血脑屏障。血脑屏障没有破裂,外周单核细胞的基因特征也没有增加,这表明ECS的作用主要是对驻留的小胶质细胞。对调控序列的无偏见分析确定了小胶质细胞视黄酸受体α(RARα)基因表达的诱导,以及多个ECS上调基因中可能存在的共同RARα结合基序。在体外研究了选择性RARα激动剂AM580对小胶质细胞对LPS反应的影响。AM580可抑制LPS诱导的细胞因子表达和活性氧的产生。利用慢性小鼠实验性自身免疫性脑脊髓炎(EAE)来证实RARα信号传导作为ECS诱导的转录途径的介质在调节小胶质细胞毒性中的作用。连续侧脑室内注射AM580可有效减轻EAE的严重程度。总之,ECS通过激活小胶质细胞视黄酸受体α途径调节中枢神经系统先天免疫系统反应,这标志着慢性神经炎症、神经精神和神经退行性疾病的一种新的治疗方法[2]。 |
| 动物实验 |
Optical recordings commenced 15–150 min after the preparation was placed in the recording chamber mounted on an Olympus BX51 microscope (upright, modified to be fixed stage, mounted on an XY platform). The recording chamber had a volume of 2 ml, a temperature of 29.5° C and was constantly superfused at a rate of 2 ml/min with preheated oxygenated (95% O2, 5% CO2) ACSF, which contained (in mM): 129 NaCl, 3 KCl, 5 KH2PO4, 25 NaHCO3, 30 d-(+)-glucose, 0.4 MgSO4 and 0.7 CaCl2. For detection of fluorescence signals, a metal halide light source PhotoFluor II or a LED light source, M470L2 (Thorlabs, New Jersey, USA) was coupled to a stereo microscope Olympus BX51 (upright, modified to be fixed stage, mounted on an XY platform) via a liquid light guide and appropriate optical filters (in nm): Biocytin-TMR, Atto 565 Biotin, Atto 565, Atto 550 Biotin, TMR; Olympus U-MWIG2, Excitation:BP520-550, DM565, Emission: BA580IF, and Atto 488 Biotin, Fluo-8 AM; Modified Olympus U-MWIB, Excitation:BP457-487, DM505, Emission: 515–550. Images were captured by an sCMOS camera controlled by the SOLIS software. Imaging was done using ×10, ×20, ×40 and ×63 water immersion objectives, at 1–10 frames/s, in image sessions lasting 1–20 s. Some Biocytin-TMR injected preparations were fixed overnight in 4% paraformaldehyde in 0.1 M Sorensen Phosphate buffer, dehydrated in increasing ethanol (20%, 30%, 50% 70%, 95%, 100%—10 min each), cleared in 100% methyl salicylate, and imaged using a Zeiss LSM 710 confocal microscope (Excitation: 561 nm, Emission: 623 nm). [1]
Brain blood barrier permeability [2] Four Biozzi ABH mice groups (3 mice per group) were evaluated; Naïve mice (negative control), EAE mice at peak of the first relapse (day 17 post-immunization, positive control), 1 h after a single ECS treatment and 24 h following 3 consecutive daily ECS treatment sessions. 1 mg of 5-(and 6)-tetramethylrhodamine biocytin (Biocytin-TMR) diluted in 100ul PBS was injected per mouse into the tail vein. Penetration into the blood circulation was indicated by pink colorization of the ears within seconds. 30 min after injection animals were anesthetized and perfused, spinal cords were removed, deep frozen and serial 10 μm longitudinal sections were prepared as described above. Nuclear counterstain was performed using DAPI (Vector Laboratories). Images of whole sections were obtained (×10 power of objective ×10 power of eyepiece) using identical laser intensity, exposure times and magnification in all cohorts. To set these parameters, livers from tracer injected mice and non-injected mice were used. |
| 参考文献 | |
| 其他信息 |
Long-range transport of biocytin has been used to trace neuronal tracts under in vivo or in vitro conditions (Kobbert et al., 2000, Thomson and Armstrong, 2011). Uptake can be facilitated by co-injection of NMDA or KCL (Chang et al., 2000, Jiang et al., 1993, Tarras-Wahlberg and Rekling, 2009, Zheng et al., 1998), but it is not entirely clear how extracellular deposited biocytin is taken up, nor have the mechanisms responsible for active transport in tracts been identified. However, the molecule appears to have affinity for some component in the transport machinery, and we have utilized this principle here to demonstrate that the fluorophore TMR coupled by a long spacer to biocytin acts as a fast retrograde tracer that can be used to label live neurons over long distances in a matter of hours.[1]
TMR biocytin had the highest transport velocity in spinal cord tracts (5.4 mm/h) of the six compounds we tested. Unconjugated TMR was not taken up into fibers, suggesting that the biocytin moiety is critical for uptake and fiber transport. Several other biotin conjugated fluorescent compounds were taken up and transported, notably Atto 488 Biotin, which was transported with a velocity close to that of TMR biocytin. Biocytin-TMR and Atto 488 Biotin have red and green emission spectra respectively and consequently can be used in dual labeling experiments. However, Atto 550 Biotin, showed very little uptake and slow transport velocities. We hypothesize that at least two chemical properties may contribute to the uptake and transport properties of the tested conjugated compounds. First, recent data show that the fluorophore moieties of the tested compounds have very different interactions with lipid bilayers, e.g. the fluorophore of the slowly transported Atto 550 Biotin interacts strongly with lipid bilayers (MIF: 33), whereas the fluorophore of Biocytin-TMR interacts weakly (MIF: 0.35,(Hughes et al., 2014). This relationship suggests that compounds with a strong lipid bilayer interaction may get trapped in lipids and cannot be recruited by the axonal transport machinery. Second, the spacer length between the fluorophore and the biotin/biocytin moiety differs among the tested compounds, i.e. TMR biocytin and Atto 488 Biotin have 20- and 18-carbon–nitrogen aliphatic spacers (Fig. 4), whereas the slower transported Atto 565 Biotin has a 13-carbon-nitrogen long aliphatic spacer. Thus, spacer length may also be a contributing factor to the transport properties of the compounds by allowing more or less space for molecular interactions necessary for transport. We have opted to perform experiments in young postnatal animals to be able to maintain large pieces of nervous tissue viable under in vitro conditions. At the oldest age (P15.5) myelination has begun, and consequently we expect TMR biocytin will also work in older adult animals, but it remains to be shown in separate experiments.[1] Several properties make TMR biocytin a very attractive neuronal tracer. The fluorophore emits light in the red end of the visual spectrum, which is an advantage since the longer emission wavelength means less scatter in the tissue thereby improving visualization of deep structures. Thus, using confocal microscopy on methyl salicylate cleared tissue we visualized Biocytin-TMR labeled fiber tracts to a depth of more than 0.5 mm (Fig. 7). Some of the best calcium sensors based on synthetic small-molecule organic dyes, e.g. Fluo-4, Fluo-8, Oregon Green, and Calcium Green have emission spectra (Em: 506–531 nm) that can be effectively separated from TMR biocytin emission (Em: 581 nm). Recently improved genetically encoded calcium sensors such as the GCaMP family also have shorter wavelength emission peaks (Em: ∼512 nm (Badura et al., 2014). Thus, neurons can be dual labeled with a calcium sensor and TMR biocytin for concurrent calcium imaging and somadendritic visualization. The duration of spontaneous calcium transients in Fluo-8 AM/TRM Biocytin labeled IO neurons was not affected by TMR biocytin, and Fluo-8 AM/TRM Biocytin labeled organotypical slice cultures showed spontaneous oscillations in dual labeled neurons. This we interpret as sign of good neuronal viability for the illumination parameters used here, i.e. low phototoxicity is expected in the use of TMR biocytin. The high transport velocity of TMR biocytin makes it possible to perform these types of experiments over the course of a few hours, as demonstrated here by imaging of oscillating commissural neurons in organotypical slice cultures containing respiratory neurons (Fig. 5). Similar experiments using Calcium Green-1 AM injections in the midline of acutely prepared brainstem slices containing oscillating commissural neurons required overnight incubation (Koshiya and Smith, 1999). Retrogradely transported TMR biocytin also Biocytin also passes into dendrites (Fig. 1, Fig. 7), which opens for experiments involving targeted dendritic recordings and dual labeling using AM dyes, which in some cases also label proximal dendrites (Del Negro et al., 2011). Juxtacellular electroporation of Biocytin-TMR leads to strong somadendritic labeling, and anterograde transport into local axonal arborisations with visible synaptic profiles within 10 min (Fig. 7). This anterograde transport could conceivable be used in uncovering local circuit motifs, targeted presynaptic experiments, and combined morphological and electrophysiological characterizations. TMR biocytin was readily taken up by cranial nerve roots (Fig. 7), and thus shows promise as an effective alternative to carbocyanine dyes such as DiO and DiI often used in developmental neurobiology to label defined cranial and spinal compartments. Finally, the presence of a lysine moiety in TMR biocytin makes it fixable with paraformaldehyde and glutaraldehyde, which aid morphological visualization in fixed and cleared tissue.[1] Using a similar rationale (Mishra et al., 2011) developed a biocytin-derived MRI contrast agent and demonstrated long-range retrograde transport of this functionalized tracer. It is not difficult to imagine how further development of the principle of a biocytin/biotin moiety coupled to a functional fluorophore may lead to reporter molecules that can be transported at high velocity in neuronal tracts and report on e.g. calcium, sodium, or chloride concentrations in synapses, dendrites, somas, or axons. However, the chemical properties of the functional fluorophore might be critical for successful transport.[1] Previous studies suggested that ECS may cause a breakdown in the blood brain barrier (BBB) (Ito et al., 2017), raising the possibility that the change in CD11b + cell expression signature might represent an influx of blood monocytes. We therefore examined the effect of ECS on the BBB by use of Biocytin-TMR tracer. First, we confirmed that systemically administered Biocytin-TMR penetrates the brain parenchyma in acute EAE, a model of neuroinflammation (Fig. 4A-D). Injection of Biocytin-TMR at one-hour post ECS and 24 h post three daily ECS sessions in naïve mice showed an intact BBB (Fig. 4E-H). We also examined whether the differentially expressed genes correspond to genes that were reported as representing infiltrating monocytes / macrophages. Only two out of 98 upregulated genes matched genes that defined infiltrating monocytes in acute EAE (Yamasaki et al., 2014) (Fig. 4I), and only three out of 98 genes matched those that defined infiltrating monocytes / macrophages in virus-induced neuroinflammation (DePaula-Silva et al., 2019). Although markers of infiltrating monocytes were described in conditions of neuroinflammation rather than in healthy CNS, these paucity of genes representing infiltrating monocytes suggest that ECS induced a phenotypic shift in resident CNS derived CD11b+ cells, rather than induced massive influx of peripheral monocytes. Single cell analysis may further reveal whether there are changes in small subsets of CNS monocular cells.[2] |
| 分子式 |
C46H60N8O7S
|
|---|---|
| 分子量 |
869.1
|
| 精确质量 |
868.43056
|
| CAS号 |
749247-49-2
|
| PubChem CID |
165412506
|
| 外观&性状 |
Typically exists as solid at room temperature
|
| LogP |
1.1
|
| tPSA |
235 Ų
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
10
|
| 可旋转键数目(RBC) |
20
|
| 重原子数目 |
12
|
| 分子复杂度/Complexity |
1710
|
| 定义原子立体中心数目 |
4
|
| SMILES |
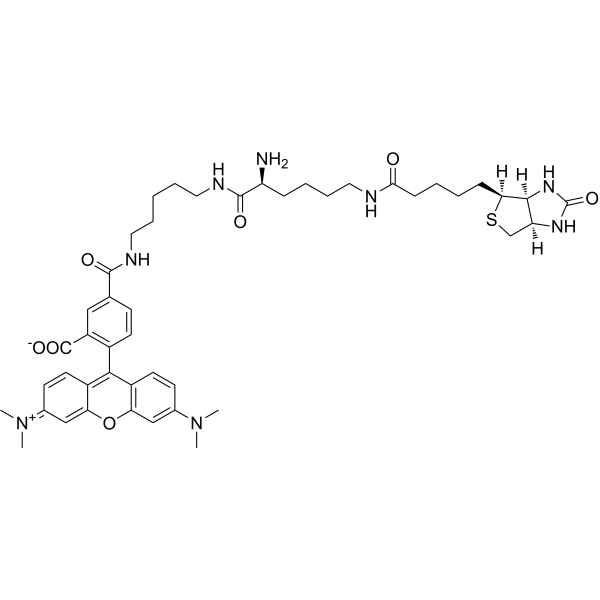
CN(C)C1=CC2=C(C=C1)C(=C3C=CC(=[N+](C)C)C=C3O2)C4=C(C=C(C=C4)C(=O)NCCCCCNC(=O)[C@H](CCCCNC(=O)CCCC[C@H]5[C@@H]6[C@H](CS5)NC(=O)N6)N)C(=O)[O-]
|
| InChi Key |
RRJNRPHRYXQIAB-VSZNSILHSA-N
|
| InChi Code |
InChI=1S/C46H60N8O7S/c1-53(2)29-16-19-32-37(25-29)61-38-26-30(54(3)4)17-20-33(38)41(32)31-18-15-28(24-34(31)45(58)59)43(56)49-22-9-5-10-23-50-44(57)35(47)12-8-11-21-48-40(55)14-7-6-13-39-42-36(27-62-39)51-46(60)52-42/h15-20,24-26,35-36,39,42H,5-14,21-23,27,47H2,1-4H3,(H5-,48,49,50,51,52,55,56,57,58,59,60)/t35-,36-,39-,42-/m0/s1
|
| 化学名 |
5-[5-[[(2S)-6-[5-[(3aS,4S,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoylamino]-2-aminohexanoyl]amino]pentylcarbamoyl]-2-[3-(dimethylamino)-6-dimethylazaniumylidenexanthen-9-yl]benzoate
|
| 别名 |
TMR Biocytin; 749247-49-2;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1506 mL | 5.7531 mL | 11.5062 mL | |
| 5 mM | 0.2301 mL | 1.1506 mL | 2.3012 mL | |
| 10 mM | 0.1151 mL | 0.5753 mL | 1.1506 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。